3. Hepatitis C
Christoph Sarrazin, Christiana Graf
Epidemiology
Global occurrence
Globally, an estimated 57 million people were living with a hepatitis C virus (HCV) infection in 2020, corresponding to 0.7% of the world’s population, with over 70% deriving from low-income and middle-income countries (Polaris Observatory HCV Collaborators 2022). Recent global estimates indicate that 30 countries account for 80% of the disease burden, with the highest prevalence being observed in countries in eastern Europe, certain countries in Africa and Asia, the Middle East and the South Caucasus and Central Africa (Spradling 2024). In contrast, HCV prevalence is observed to be low with <1.0% in most developed countries. Over the past 5 years a considerable decline of 6.8 million HCV infections was observed (Polaris Observatory HCV Collaborators 2022). However, these estimates may rather derive from revised results of prevalence data than from the elimination progress, although country-specific therapeutic and harm reductions programmes have also contributed to a substantial decline (e. g. Egypt)(Polaris Observatory HCV Collaborators 2022, Polaris Observatory HCV Collaborators 2017).
Currently, about 1.5 million new HCV infections are estimated each year with injecting drug use and unsafe health-care injections accounting for most cases (Polaris Observatory HCV Collaborators 2017). Overall, epidemiology of HCV is rapidly changing due to a scale up in screening and prevention measures and high cure rates in the era of interferon free direct acting antiviral (DAA) treatment. The implementation of a routinely screening of donated blood for bloodborne viruses in the early 1990, high-coverage needle and syringe programmes as well as opioid agonist therapy have led to a significant reduction of HCV infections in people who inject drugs (PWID) and of transfusion associated HCV infections. Accordingly, a peak of annual HCV incidence was observed in most countries between 1970 and 2005 followed by a decline in PWIDs in many high-income countries (Morris 2017). Nevertheless, in the USA and some low-income and middle-income countries a sustained high or even increasing incidence has been reported in the last years (Artenie 2023, Liang and Ward 2018, Trickey 2019).
HCV strains are classified into eight major genotypes, with at least 86 subtypes identified to date, whose prevalence and distribution vary considerably between different regions (Borgia et 2018, Polaris Observatory HCV Collaborators 2017). HCV subtypes 1a and 1b are the most common in Northern America, Europe and Japan, while genotype 2 accounts for most infections in West Africa and in South America (Gower 2014, Messina 2015, Petruzziello 2016). Subtype 3a, which is very common among intravenous drug abusers, is common mainly in Europe, USA, Pakistan and South East Asia, while genotype 4 prevails in North Africa and in the Middle East and genotypes 5 and 6 are endemic, respectively, in South Africa and in South China / South East Asia (Gower 2014, Messina 2015, Petruzziello 2016, Polaris Observatory HCV Collaborators 2017, Zhang 2017).
Transmission
Parenteral exposure to HCV is the most efficient means of transmission. Most common routes include transfusion of unscreened blood products, injection drug use and unsafe skin-penetrating health-care practices. Infrequent modes of transmission are vertical and heterosexual transmission.
It is estimated that most recently acquired infections occur in individuals who have injected illicit drugs. However, HCV infection has also been associated with a history of injecting recreational drugs such as methamphetamine in a sexual context or intranasal cocaine use, presumably due to blood on shared straws or other sniffing paraphernalia. Besides recreational drug use, sexual risk behaviour represents the predominant risk factor for HCV transmission in men who have sex with men (MSM), with increased risk in men with human immunodeficiency virus (HIV) coinfection. In the last decades, observed outbreaks of recently acquired HCV infections in several cities in Europe and the United States among MSM have focused attention on sexual transmission of HCV (Boesecke 2015, Boesecke 2012). Sexual behaviors with HCV acquisition in this population including fisting, anal intercourse without condom, group sex, having many sex partners in a short time period and mucosal damage have been identified as primary risk factors for HCV transmission in MSM (Bradshaw et al., 2020; Newsum et al., 2021). In contrast, HCV transmission by sexual contact is uncommon between heterosexual couples (<0.1% per year in monogamous heterosexual couples)(Terrault 2013). Perinatal transmission of HCV is observed in about 5% of infants born to women with HCV, with increased risk associated with maternal HIV co-infection (10%), higher maternal HCV RNA (≥6.0 log10 IU/mL), amniocentesis, prolonged rupture of membranes and invasive fetal monitoring (Ades 2023, Benova 2014, Deng 2023, Kushner 2022, Ohto 1994, Terrault 2021).
In high-income countries, PWIDs and HIV positive MSM represent the populations at highest risk to acquire HCV infections (Degenhardt 2017, Jin 2010). In middle-income and low-income countries, unsafe health medical procedures are the most commonly identified source of infection, with an increasing burden related to injection drug use.
Clinical presentation and natural history of HCV infection
Recently acquired HCV infection
Most people (>70%) have no symptoms attributable to recently acquired HCV infection, making early diagnosis challenging (Vogel 2009). Symptoms associated with recently acquired infection include jaundice, fever, headache, malaise, anorexia, nausea, vomiting, diarrhoea, and abdominal pain. Aminotransferases become elevated approximately 6-12 weeks after exposure. The elevation of aminotransferases can have a broad range among individuals but tends to be more than 10-30 times the upper limit of normal. HCV antibodies can be found about 6-8 weeks after exposure in most cases. However, in some patients HCV seroconversion can be delayed. Thus, if recently acquired HCV infection is suspected, HCV-RNA testing by PCR is recommended as HCV antibodies might not present yet. (Hajarizadeh et al., 2015). Periodic screening for infection may be warranted in certain groups of patients who are at high risk for infection, e. g. HIV positive MSM or persons who use drugs.
Although most people have viral persistence and develop chronic HCV infection, some undergo spontaneous clearance (15–35%), usually within 6 months (Aisyah 2018, Ingiliz 2017, Micallef 2006). Factors, that have been found to be associated with spontaneous clearance of HCV infection, were associated with female gender, younger age at infection, lower HCV RNA load and co-infection with hepatitis B virus (HBV)(Grebely 2014, Martinello 2018, Shin 2016). Immunodeficiency has been observed to reduce the chance of spontaneous clearance (<20%)(Aisyah 2018, Ingiliz 2017).
Introduction of highly efficient DAA agents has led to several changes in management and treatment of patients with recently acquired HCV infections with varying recommendations of international guidelines (EASL 2020, AASLD 2023, EACS 2024). Treatment initiation 4 weeks after HCV has been diagnosed and after spontaneous seroconversion has been ruled out, is recommended by the European AIDS Clinical Society (EACS) guideline and has been shown to be beneficial for patients’ outcome, to reduce transmission and to be cost effective (EACS 2024).
Chronic HCV infection
In most individuals chronic HCV infection causes progressive disease, that deteriorate from chronic inflammation to fibrosis and liver cirrhosis. Approximately, 20-30% of chronically infected patients develop liver cirrhosis over a period of 20 to 30 years (Freeman 2001, Thein 2008). It is not clear why HCV results in chronic infection in most cases. The rapid mutation of the virus and its high genetic diversity may allow HCV to escape immune recognition. Host factors such as HCV-specific CD4 T cell and NK cell responses, IL28B gene polymorphisms and specific HLA-DRB1 alleles have been shown to be involved in the ability to spontaneously clear the virus (Lauer and Walker 2001, Rauch 2010, Thomas 2009).
Once liver cirrhosis has been diagnosed, the risk of hepatic decompensation and hepatocellular carcinoma is about 3% and 1-2% per year, respectively (Fattovich 1997). Factors associated with increased risk of hepatocellular carcinoma are elevated bilirubin, male gender, markers of advanced liver cirrhosis and portal hypertension as well as prolonged prothrombin time and thrombocytopenia (Villanueva 2019). Moreover, about 30% to 40% of individuals with chronic HCV infection develop extrahepatic manifestations and diseases such as mixed cryoglobulinemic vasculitis, porphyria cutanea tarda, lichen planus and B-cell non-Hodgkin lymphoma (Zignego and Craxì 2008).
Diagnosis
The standard algorithm for testing HCV involves a two-step process. Serologic tests are sufficient when chronic hepatitis C is expected, with a sensitivity of more than 99% with currently used 3rd generation assays. Positive serologic results require HCV ribonucleic acid (RNA) or with slightly reduced sensitivity HCV core antigen measurement in order to differentiate between chronic hepatitis C and resolved HCV infection in the past. Anti-HCV antibodies are usually detectable within 6 weeks after exposure, although in severely immunocompromised individuals, their detection may be delayed or absent (Netski 2005). Thus, when recently acquired hepatitis C is considered, serologic screening alone is insufficient because anti-HCV antibodies may develop late after transmission of the virus. In contrast, HCV RNA is detectable within a few days of infection, making nucleic acid-based tests mandatory in diagnosing recently acquired hepatitis C. HCV testing is usually conducted by collecting a blood sample and analysing it in a centralised laboratory. The complexity and costs associated with HCV diagnostics pose challenges to large-scale testing. Simplifying the diagnostic process and utilising easily accessible samples could improve testing uptake and enable decentralised care, especially among low-income settings and in key populations. Various approaches, such as dried blood spot testing, point-of-care antibody and RNA testing and reflex RNA testing from HCV antibody positive samples have demonstrated effectiveness in enhancing testing uptake and diagnosis (Cunningham 2022). Point-of-care HCV testing has simplified testing algorithms, increased diagnosis rates, and facilitated linkage to care and treatment. At the point of care, antibody testing can be conducted using fingerstick blood, whole blood, or oral fluid samples, providing results in less than 20 minutes. Similarly, HCV RNA testing can be performed using fingerstick or whole blood samples, with results available within 1 hour. These point-of-care tests have shown excellent diagnostic performance in various populations and settings, including community health centres, drug treatment clinics, prisons, homelessness settings, supervised drug consumption rooms, residential rehabilitation facilities as well as in countries with restricted health care resources.
Management of HCV infection
Indications for treatment: who should be treated?
Generally, all treatment-naïve and treatment-experienced patients with recently acquired or chronic HCV infection should be considered for HCV treatment, because cure of infection is associated with reductions in the risk of hepatocellular carcinoma (HCC), liver-related and all-cause mortality, improvements in liver fibrosis and quality of life. One further reason for early treatment initiation is the prevention of further HCV transmission especially in patients with high risk of transmitting HCV (PWIDs, MSM with high-risk sexual behaviour, women of childbearing age and prison inmates). Besides early HCV treatment in PWIDs and MSM with high-risk sexual practices, constructive preventive strategies such as raising awareness as well as behavior interventions are necessary to prevent reinfections and further HCV transmission.
Patients with significant liver fibrosis (METAVIR score F2 or F3) or liver cirrhosis (METAVIR score F4), including those with decompensated liver cirrhosis, should be considered for urgent treatment initiation. Further reasons for prompt treatment initiation are clinically significant extrahepatic manifestations (e. g. HCV immune complex-mediated vasculitis, HCV infection related B-cell non-Hodgkin lymphomas (B-NHL), HCV recurrence during or after liver transplantation, patients at risk of rapid progression of liver disease due to concomitant diseases (e. g. patients with coinfections such as HBV or HIV or in recipients of solid organs or stem cells).
Endpoint of HCV therapy
The goal of antiviral therapy is to cure hepatitis C via a sustained elimination of the virus. Sustained virologic response (SVR) is the established efficacy endpoint and is defined as undetectable HCV RNA in serum or plasma 12 (SVR12) or 24 (SVR24) weeks after the end of treatment.
In settings, where HCV RNA assays are not available or not affordable, using a HCV core antigen assay with a lower limit of detection corresponding to approx. 4.000 IU/mL HCV RNA can be used as an alternative endpoint. Long-term follow-up studies have shown that in most cases SVR corresponds to a definitive cure of HCV infection (Frías 2019, Sarrazin 2017).
Pretherapeutic assessment of patients
When assessing individuals with hepatitis C (HCV) infection, several key factors should be considered.
Evaluation of liver disease severity is crucial prior to treatment initiation in order to identify the presence or absence of liver cirrhosis or advanced liver fibrosis (METAVIR score F3), because some treatment regimens must be adjusted and post-treatment prognosis as well as surveillance for HCC are dependent on the severity of liver disease. Non-invasive tools should be preferred over liver biopsy to assess advanced liver disease. Liver stiffness measurement obtained with Transient Elastography (TE), point-shear wave elastography (pSWE) or 2D-SWE are well validated tools to determine significant fibrosis or liver cirrhosis (Berzigotti 2021). If possible, liver stiffness measurement should be performed in combination with blood biomarkers such as the aspartate aminotransferase to plated ratio index (APRI) and fibrosis-4 score (FIB-4) in order to improve accuracy (Castéra 2010, Castéra 2005). The need for liver biopsy prior to HCV treatment has become rare and indication to liver biopsy is limited to cases of suspected mixed etiologies (e. g. metabolic syndrome or autoimmunity).
Moreover, relevant comorbidities such as HIV-, or HBV coinfection, renal insufficiency and further causes of liver disease (e. g. metabolic-associated fatty liver disease or alcoholic liver disease) should be systematically investigated.
Beyond that, assessment of factors associated with HCV transmission such as substance abuse or sexual risk behavior and factors associated with liver disease progression, including alcoholic use, obesity and diabetes mellitus should be performed.
Prior to initiating HCV therapy, the presence of viraemia should be verified by detecting HCV RNA or if not available or not affordable HCV core antigen in serum or plasma. Identifying HCV genotype is not mandatory before starting pan-genotypic HCV drug regimens but should be done prior to initiating genotype-specific DAA therapy. In addition, HCV genotype determination is useful, if available, in order to identify HCV subtypes, which are resistant to NS5A inhibitors (i. e. HCV genotype 3c), and to identify patients, who may benefit from an adapted HCV treatment. Figure 1 provides an overview of the treatment process in the case of HCV therapy, from pre-treatment assessment to a simplified therapy with genotype/subtype-free combinations and the post-treatment follow-up.
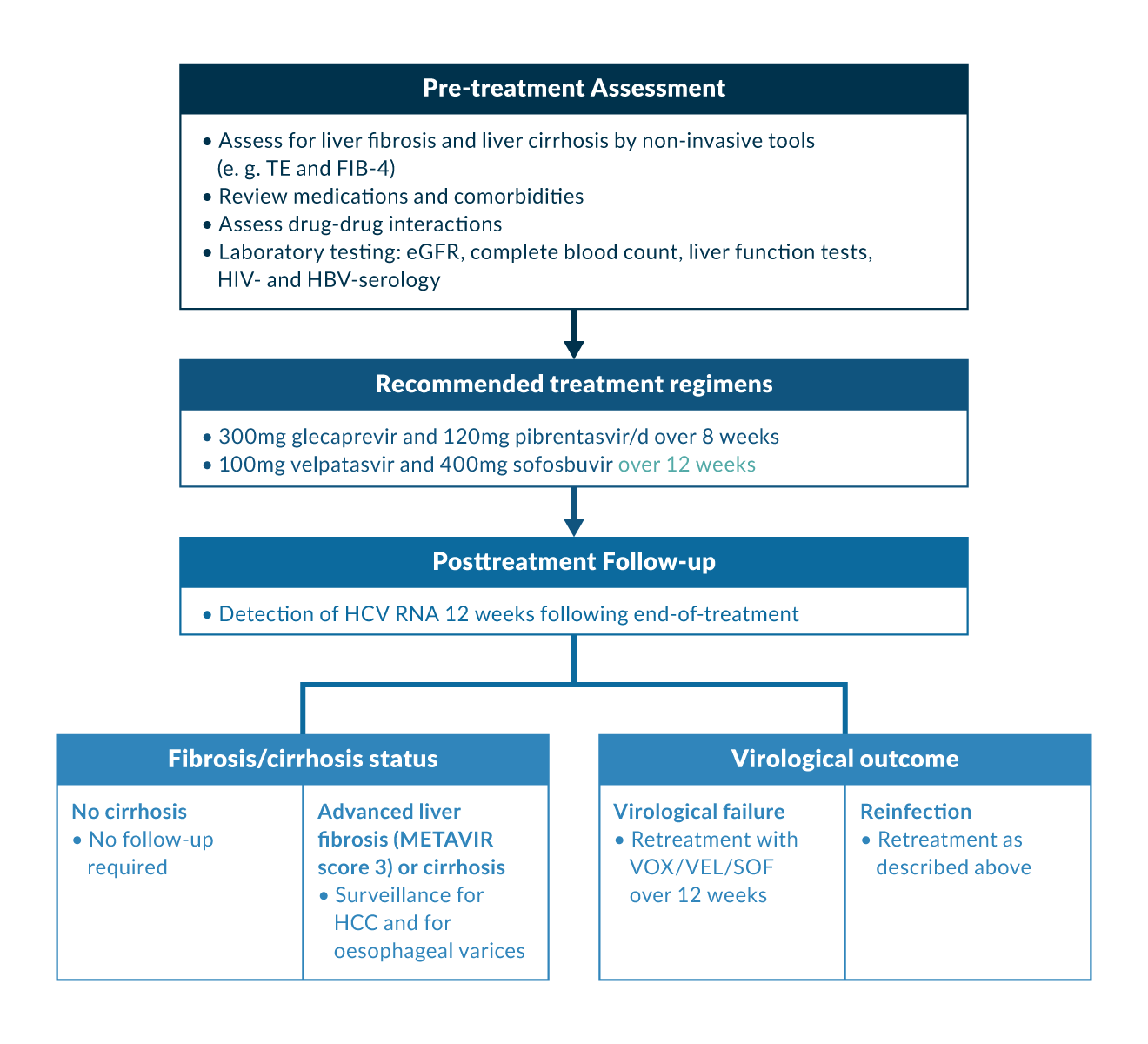 Figure 1. Simplified genotyping-free algorithm for HCV treatment among treatment-naive patients with and without cirrhosis
Figure 1. Simplified genotyping-free algorithm for HCV treatment among treatment-naive patients with and without cirrhosis
Direct-acting antiviral therapy
Continuous research on the HCV life cycle enabled the development of a new generation of antiviral substances for treating HCV infection, the direct acting agents (DAAs). In contrast to the rather non-specific treatment with pegylated interferon (peg IFN-α) and ribavirin (RBV), DAAs inhibit specific viral proteins necessary for HCV replication. Based on their molecular mode of action DAAs are classified in NS3/4 protease inhibitors, that prevent the proteolytic processing of the HCV polyprotein between NS3 and NS4A, non-nucleoside and nucleotide analogue NS5B RNA-dependent RNA-polymerase inhibitors, which target the NS5B, and NS5A inhibitors, that bind to the NS5A domain 1 and prevent RNA from binding, therefore disrupting RNA replication (Gottwein 2018, Powdrill 2010). The introduction of these IFN-free DAAs has revolutionised and simplified clinical management in the past decade.
With the approval of the nucleotide analogue sofosbuvir in December 2013 in the USA and in January 2014 in Europe, the first IFN-free therapy became widely available. The first interferon-free regimens were the dual combinations of ledipasvir-sofosbuvir (LDV/SOF) and sofosbuvir plus simeprevir, which were approved for genotype 1 HCV infection 2014. With the approval of the fixed-dose combination of the second-generation pan-genotypic NS5A inhibitor velpatasvir with sofosbuvir in summer 2016, the first pan-genotypic fixed-dose combination regimen was available. Today, four pangenotypic fixed-dose combination regimens are available: velpatasvir-sofosbuvir (VEL/SOF), daclatasvir-sofosbuvir (DAC/SOF), glecaprevir/pibrentasvir (GLE/PIB) and voxilaprevir/velpatasvir/sofosbuvir (VOX/VEL/SOF)(Bourlière 2017, Brown 2020, Feld 2015, Foster 2015, Kwo 2017, Sulkowski 2014, Wyles 2017, Zeuzem 2018). The triple fixed-dose dose combination of VOX/VEL/SOF was approved in 2017 and enables re-treatment of patients failing DAA therapy. Moreover, the non-pangenotypic combination of grazoprevir and elbasvir is possible in settings, where available HCV genotype and subtype determination enables the identification of patients infected with HCV genotype 1b (Jacobson 2017). Table 1 and 2 give an overview of the recommended first-line treatment schedules and treatment durations in patients with compensated liver disease, depending on whether genotype/subtype determination is available. All approved IFN-free DAAs have an excellent safety and efficacy profile (SVR≥95%, including patients with compensated liver cirrhosis), short treatment duration and low resistance-related failure.
Table 1. Overview of genotyping/subtyping-free antiviral combinations in DAA-naïve patients with compensated liver disease| Genotype | Cirrhosis status | Prior treatment | Glecaprevir-pibrentasvir | Velpatasvir-sofosbuvir |
| All genotypes | No cirrhosis | Treatment-naïve | 8 weeks* | 12 weeks** |
| Peg-IFN+RBV | ||||
| Compensated cirrhosis | Treatment-naïve | |||
| Peg-IFN+RBV | 12 weeks* |
*In cases of HCV GT3 and treatment experience meaning pre-treatment with (PEG-) interferon ± ribavirin, sofosbuvir with (PEG-)interferon + ribavirin or sofosbuvir + ribavirin, extended treatment duration over 16 weeks is recommended.
**In patients with liver cirrhosis, additional treatment with ribavirin should be considered. Table 2. Overview of antiviral combinations in DAA-naïve patients with compensated liver disease if genotype is available
| Genotype | Cirrhosis status | Prior treatment | Glecaprevir-pibrentasvir | Sofosbuvir-velpatasvir | Grazoprevir-elbasvir | Voxilaprevir-velpatasvir- sofsobuvir |
| 1b | No cirrhosis | Treatment-naïve | 8 weeks | 12 weeks* | 12 weeks | No |
| Peg-IFN+RBV | ||||||
| Compensated cirrhosis | Treatment-naïve | |||||
| Peg-IFN+RBV | 12 weeks | |||||
| 1a, 2, 4, 5, 6 | No cirrhosis | Treatment-naïve | 8 weeks | 12 weeks* | No | No |
| Peg-IFN+RBV | ||||||
| Compensated cirrhosis | Treatment-naïve | |||||
| Peg-IFN+RBV | 12 weeks | |||||
| 3 | No cirrhosis | Treatment-naïve | 8 weeks | 12 weeks* | No | No |
| Peg-IFN+RBV | 12-16 weeks | No | ||||
| Compensated cirrhosis | Treatment-naïve | 8 weeks | 12 weeks* | 12 weeks | ||
| Peg-IFN+RBV | 16 weeks | 12 weeks |
**In patients with liver cirrhosis, additional treatment with ribavirin should be considered.
If genotype determination is not available or affordable, simplified treatment algorithms are feasible in most cases: the only information needed to start treatment with the genotyping/subtyping-free treatment regimens VEL/SOF or GLE/PIB in treatment-naïve patients with compensated liver disease (no liver cirrhosis or compensated liver cirrhosis Child-Pugh A), who are treatment-naïve or treatment-experienced with an IFN-based regimen, is the presence of HCV replication and possible drug-drug interactions. Evidence from several clinical trials as well as real-world studies exists and supports that treatment with GLE/PIB over 8 weeks or VEL/SOF over 12 weeks is effective if genotype/subtype determination is not available (EASL 2020)(Table 1). However, in many middle- and low-income countries, the recommended pan-genotypic DAA combinations are not available. In these cases, the generic combination of daclatasvir and sofosbuvir (DAC/SOF) is safe and provides high SVR rates at a relatively low price (Pawlotsky 2020). If this combination is also not accessible, the use of older DAA combinations or, in rare cases, treatment with IFN-based therapy is necessary (Zeng 2020). For detailed information on older treatment regimens such as the dual treatment with Peg-IFN+RBV and triple treatment regimens including Peg-IFN+RBV plus protease inhibitors, we refer to the previous edition of the textbook dating from 2015. For detailed information on older DAA combinations sofosbuvir + ribavirin, simeprevir + sofosbuvir, daclatasvir + sofosbuvir and for the 3D combination (ombitasvir, paritaprevir/r + dasabuvir) we refer to the textbook dating from 2016.
Management of HCV in special epidemiological groups
HCV treatment in children and adolescents
A systematic review updated in 2016 on the prevalence of HCV viraemia in children and adolescents aged 1-19 years, revealed an overall burden of 3.5 million cases or 0.15% of the global population (Indolfi 2019). Clinical trial data evaluating DAA regimens in children and adolescents have allowed the expanded use of these safe and well-tolerated HCV therapies in the paediatric population. Generally, HCV treatment in children and adolescents is based on the recommendations for adults.
Based on representative study results, GLE/PIB was approved as a pan-genotypic therapy for children and adolescents in 2019 (Jonas 2020). For children aged 12 and over, the effectiveness, dosage and treatment duration of therapy correspond to those approved for adults. Regarding the administration of GLE/PIB in children aged 3-11 years, dosage adjustment is required depending on age and body weight (Table 3). Based on positive study results, VEL/SOF has also been approved for paediatric patients aged ≥3 years in June 2021 (Jonas 2019). The recommended dose of VEL/SOF in patients aged 3 to less than 18 years is based on weight. Following positive results of two clinical trials, genotype-specific therapy of LED/SOF was approved for paediatric patients aged 3 years and older infected with HCV genotype 1, 4, 5 and 6 (Murray 2018, Schwarz 2020). DAC/SOF is not approved in children and adolescents but is recommended by WHO based on real-world data and pharmacokinetic modelling for the use in this population in low-income and middle-income countries (Pawlotsky 2020).
Table 3. Overview of genotyping/subtyping-free antiviral combinations in DAA-naïve patients with compensated liver disease| Treatment regimen | Usual dose |
| GLE + PIB | |
| Adults and adolescents (≥12 years) ≥ 45kg | 300mg GPR + 120mg PBR/day in 1 dose |
| Children (3-11 years) ≥ 30 to < 45kg | 250mg GPR and 100mg PBR/day in 1 dose |
| Children (3-11 years) ≥ 20 to < 30kg | 200mg GPR and 80 PBR/day in 1 dose |
| Children (3-11 years) < 20 kg | 150mg GPR and 60mg PBR/day in 1 dose |
| VEL + SOF | |
| Adults and adolescents (≥12 years) ≥30 kg | 100mg VEL + 400mg SOF/day in 1 dose |
| Children (3-11 years) ≥17 to <30 kg | 50mg VEL + 200mg SOF/day in 1 dose |
| Children (3-11 years) <17 kg | 37.5mg VEL + 150mg SOF/day in 1 dose |
| GZR + ELB | |
| Adults and adolescents (≥12 years) ≥30 kg | 100mg GZR + 50mg ELB/day in 1 dose |
| VOX + VEL + SOF | |
| Adults and adolescents (≥12 years) ≥ 45kg | 100mg VOX + 100mg VEL + 400mg SOF/day in 1 dose |
HCV treatment in pregnancy
Data situation is still limited regarding the teratogenic risk of DAAs. Thus, safe contraception should be recommended during antiviral treatment. Antiviral therapy during pregnancy and breastfeeding is currently not recommended. However, real world data on different DAA regimens (i.e. VEL/SOF, DAC/SOF) used in pregnancy showed no adverse effects on pregnancies and newborns and a prospective study for the use of VEL/SOF during pregnancy is ongoing (AbdAllah 2021, Ades 2023, Chappel). In HCV-monoinfected patients, the risk of vertical transmission is approx. 5%, a caesarean section does not reduce the risk of transmission (Yeung 2014). HCV-infected mothers are not advised against breastfeeding. Diagnosis of HCV infection in newborns is uncertain during the first weeks and spontaneous resolution is not infrequent until the age of 3 years.
HCV treatment in people with hepatocellular carcinoma
In patients with chronic hepatitis C and hepatocellular carcinoma (HCC), a coordinated approach and multidisciplinary tumour board decision is required. The indication for antiviral therapy should be made individualised in an experienced centre, taking into account tumour stage, treatment concept and the overall prognosis. If a curative treatment approach exists for HCC, antiviral therapy is generally indicated in those patients. DAA interactions with immunotherapies for hepatocellular carcinoma are not a concern. However, in contrast to HBV-associated HCC, where antiviral suppression therapy has a clinical significance in palliative treatment, no reliable and confirmed analogous data exist for the palliative treatment of HCV-associated HCC (Zhang and Guo 2015).
Post-treatment surveillance
After achieving SVR12, patients with normal liver enzymes and without advanced liver disease (advanced liver fibrosis METAVIR F3 or liver cirrhosis) require no further follow-up. HCV infection can be considered as definitely cured in these patients.
Patients with persistently elevated liver parameters post SVR12 should be examined for further hepatopathies. Individuals with advanced liver fibrosis (METAVIR score F3) or liver cirrhosis (F4) should remain under surveillance for HCC by ultrasound and for clinically significant portal hypertension. Long-term post-SVR follow-up studies revealed that the risk of developing HCC is significantly reduced compared to untreated patients post SVR but it remains (Arase 2013, Carrat 2019, Nahon 2017, van der Meer 2012). Thus, duration of HCC surveillance in patients with advanced fibrosis or liver cirrhosis is indefinite despite SVR and potential normalisation of non-invasive liver fibrosis assessment tools. However, a recent meta-analysis showed good correlation between declined values for transient elastography 24 weeks after the end-of-treatment and a lower risk for HCC development, although a specific cut-off cannot be determined so far (Esposto 2024). In line with these results, discontinuation of surveillance for clinically significant portal hypertension can be considered if improvement can be observed following SVR (liver stiffness measurement <12 kPa and platelet count >150x109 /L)(de Franchis 2022, Semmler 2024).
Remaining challenges
Tremendous progress in DAA therapy, that resulted in pan-genotypic fixed-dose combinations, solved most of the remaining challenges in anti-HCV treatment. Today, IFN-free DAA combinations enable HCV cure quite easily and safe without any relevant adverse effects. However, some patients still fail to cure.
Treatment of patients with virological failure after pan-genotypic DAA therapy
With currently available highly efficacious pangenotypic DAA regimens, treatment failure is rare. However, some difficult-to-treat subgroups remain, who fail not only first-line therapy but also retreatment with VOX/VEL/SOF (Bourlière 2017, Vermehren 2020). Studies have shown that virologic treatment failure to VOX/VEL/SOF is primarily observed in patients with difficult-to-treat cofactors such as HCC, liver cirrhosis and HCV genotype 3 (Degasperi 2019, Graf 2024, Llaneras 2019). In contrast, clinical trials as well as real-world studies have shown that RASs as well as rare genotypes and chimera have no impact on cure in patients retreated with VOX/VEL/SOF (Bourlière 2017, Degasperi 2019, Graf 2024, Llaneras 2019).
In these cases, rescue therapy with GLE/PIB+SOF over 24 weeks or retreatment with VOX/VEL/SOF + RBV over 16-24 or weeks is recommended (Pawlotsky 2020). However, only limited clinical experience consisting of case series involving fewer than 25 patients supports this recommendation (Bernhard and Stickel 2020, Dietz 2021, Fierer and Wyles 2020, Martin 2021).
Treatment of patients with decompensated liver disease
Patients with decompensated liver cirrhosis including those after TIPS implantation represent a further subgroup which is still difficult to treat even in the age of DAAs. Due to its hepatic metabolisation, NS3/4 protease inhibitors are contraindicated in these patients, which limits treatment option to NS5A inhibitors, sofosbuvir and RBV. Thus, the European Association for the Study of the Liver (EASL) recommends the combination of VEL/SOF over 12 weeks as the treatment of choice in patients with decompensated liver cirrhosis (Child Pugh B or C) or with compensated liver cirrhosis (Child Pugh A) and prior episodes of decompensation (Pawlotsky 2020).
This recommendation is based on the results of the ASTRAL-4 study, which demonstrated high SVR rates in patients with a Child Pugh class B liver cirrhosis treated with VEL/SOF with weight-based RBV (Curry 2015). Due to the observed benefit of adding RBV, add-on administration should be started at a dose of 600mg in patients with decompensated liver cirrhosis and should be adjusted depending on tolerance (Curry 2015, Lu 2019). In cases of contraindications of the use of RBV or poor tolerance to RBV on treatment, the fixed dose combination of VEL/SOF should be administered over 24 weeks.
It is still not clear until which stage of liver cirrhosis patients benefit from an antiviral treatment and when to defer HCV treatment to after liver transplantation (Samur 2018). Several studies assessed whether achieving SVR prior to liver transplantation would lead to a benefit for patients with decompensated liver cirrhosis. Most of them demonstrated that HCV cure led to substantial improvement in liver function, portal hypertension and potential delisting in patients with a MELD <20 (Deterding 2015, El-Sherif 2018, Mandorfer 2016). Data on HCV patients with a MELD score >20 are rare, but existing studies demonstrate that above a MELD of 20 the risk of adverse events and death during treatment was higher and life expectancy benefit of treating was less than 1 year. Moreover, the likelihood of substantial enhancement in liver function and removal from the transplant list was minimal at this stage of disease was minimal. Accordingly, studies have shown that pre-liver transplantation treatment was only observed to be cost-effective for patients with a MELD score <20 (Chhatwal 2017, Cortesi 2018). Accordingly, current EASL guidelines recommend HCV treatment of patients with decompensated liver cirrhosis (Child Pugh B or C) and a MELD score < 18-20 prior to liver transplantation.
Treatment of patients with rare HCV subtypes
Due to a reduced susceptibility to current DAA therapies, some HCV sub-types represent a further challenge in treating and eliminating HCV infection. The hepatitis C virus is incredibly diverse, with subtypes distributed variably around the world. These viral genotypes fall into two categories: epidemic subtypes, which are prevalent globally, and endemic subtypes, mainly found in Africa and Asia. The high genetic diversity of endemic strains raises the possibility of resistance to pan-genotypic direct-acting antiviral regimens. While many endemic subtypes respond well to these therapies, others (such as genotypes 1l, 3b, and 4r) do not perform as predicted (Dietz 2024, Wei 2020, Wei 2019). Several genotypes, rare in high-income countries but common elsewhere, have not yet undergone comprehensive clinical trials. Further sequencing and clinical studies in sub-Saharan Africa and Asia are essential to monitor treatment response and support the World Health Organization’s 2030 elimination strategy.
References
AbdAllah, M., Alboraie, M., Abdel-Razek, W., et al. 2021. Pregnancy outcome of anti-HCV direct-acting antivirals: Real-life data from an Egyptian cohort. Liver Int. Off. J. Int. Assoc. Study Liver 41, 1494–1497.
Ades, A.E., Gordon, F., Scott, K., et al. 2023. Spontaneous Clearance of Vertically Acquired Hepatitis C Infection: Implications for Testing and Treatment. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 76, 913–991.
Aisyah, D.N., Shallcross, L., Hully, A.J., O’Brien, A., Hayward, A., 2018. Assessing hepatitis C spontaneous clearance and understanding associated factors-A systematic review and meta-analysis. J. Viral Hepat. 25, 680–698.
Arase, Y., Kobayashi M., Suzuki, F., et al. 2013. Effect of type 2 diabetes on risk for malignancies includes hepatocellular carcinoma in chronic hepatitis C. Hepatol. Baltim. Md 57, 964–973.
Artenie, A., Stone, J., Fraser, H., et al., HIV and HCV Incidence Review Collaborative Group, 2023. Incidence of HIV and hepatitis C virus among people who inject drugs, and associations with age and sex or gender: a global systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 8, 533–552.
Benova, L., Mohamoud, Y.A., Calvert, C., Abu-Raddad, L.J., 2014. Vertical transmission of hepatitis C virus: systematic review and meta-analysis. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 59, 765–773.
Bernhard, B., Stickel, F., 2020. Successful fourth line treatment of a relapse patient with chronic hepatitis C virus infection genotype 3a using sofosbuvir, glecaprevir/pibrentasvir, and ribavirin: a case report. Z. Gastroenterol. 58, 451–455.
Berzigotti, A., Tsochatzis, E., Boursier, J., et al. 2021. EASL Clinical Practice Guidelines on non-invasive tests for evaluation of liver disease severity and prognosis – 2021 update. J. Hepatol. 75, 659–689.
Boesecke, C., Grint, D., Soriano, V., et al. 2015. Hepatitis C seroconversions in HIV infection across Europe: which regions and patient groups are affected? Liver Int. Off. J. Int. Assoc. Study Liver 35, 2384–2391.
Boesecke, C., Wedemeyer, H., Rockstroh, J.K., 2012. Diagnosis and treatment of acute hepatitis C virus infection. Infect. Dis. Clin. North Am. 26, 995–1010.
Borgia, S.M., Hedskog, C., Parhy, B., et al. 2018. Identification of a Novel Hepatitis C Virus Genotype From Punjab, India: Expanding Classification of Hepatitis C Virus Into 8 Genotypes. J. Infect. Dis. 218, 1722–1729.
Bourlière, M., Gordon, S.C., Flamm, S.L., et al., POLARIS-1 and POLARIS-4 Investigators, 2017. Sofosbuvir, Velpatasvir, and Voxilaprevir for Previously Treated HCV Infection. N. Engl. J. Med. 376, 2134–2146. https://doi.org/10.1056/NEJMoa1613512
Bradshaw, D., Vasylyeva, T.I., Davis, C., et al. 2020. Transmission of hepatitis C virus in HIV-positive and PrEP-using MSM in England. J. Viral Hepat. 27, 721–730.
Brown, R.S., Buti, M., Rodrigues, L., et al. 2020. Glecaprevir/pibrentasvir for 8 weeks in treatment-naïve patients with chronic HCV genotypes 1-6 and compensated cirrhosis: The EXPEDITION-8 trial. J. Hepatol. 72, 441–449.
Carrat, F., Fontaine, H., Dorival, C., et al., French ANRS CO22 Hepather cohort, 2019. Clinical outcomes in patients with chronic hepatitis C after direct-acting antiviral treatment: a prospective cohort study. Lancet Lond. Engl. 393, 1453–1464.
Castéra, L., Sebastiani, G., Le Bail, B., de Lédinghen, V., Couzigou, P., Alberti, A., 2010. Prospective comparison of two algorithms combining non-invasive methods for staging liver fibrosis in chronic hepatitis C. J. Hepatol. 52, 191–198.
Castéra, L., Vergniol, J., Foucher, J., et al. 2005. Prospective comparison of transient elastography, Fibrotest, APRI, and liver biopsy for the assessment of fibrosis in chronic hepatitis C. Gastroenterology 128, 343–350.
Chappell et al., Safety, tolerability and outcomes of sofosbuvir/velpatasvir in treatment of chronic hepatitis C virus during pregnancy: interim results from the STORC Study. AASLD 2023; #LB5018
Chhatwal, J., Samur, S., Kues, B., et al. 2017. Optimal timing of hepatitis C treatment for patients on the liver transplant waiting list. Hepatology 65, 777.
Cortesi, P.A., Belli, L.S., Facchetti, R., et al., the European Liver and Intestine Transplant Association (ELITA), 2018. The optimal timing of hepatitis C therapy in liver transplant-eligible patients: Cost-effectiveness analysis of new opportunities. J. Viral Hepat. 25, 791–801.
Cunningham, E.B., Wheeler, A., Hajarizadeh, B., et al. 2022. Interventions to enhance testing, linkage to care, and treatment initiation for hepatitis C virus infection: a systematic review and meta-analysis. Lancet Gastroenterol. Hepatol. 7, 426–445.
Curry, M.P., O’Leary, J.G., Bzowej, N., et al., ASTRAL-4 Investigators, 2015. Sofosbuvir and Velpatasvir for HCV in Patients with Decompensated Cirrhosis. N. Engl. J. Med. 373, 2618–2628.
de Franchis, R., Bosch, J., Garcia-Tsao, G., Reiberger, T., Ripoll, C., Baveno VII Faculty, 2022. Baveno VII - Renewing consensus in portal hypertension. J. Hepatol. 76, 959–974.
Degasperi, E., Spinetti, A., Lombardi, A., et al., NAVIGATORE-Lombardia and Veneto Study Groups, 2019. Real-life effectiveness and safety of sofosbuvir/velpatasvir/voxilaprevir in hepatitis C patients with previous DAA failure. J. Hepatol. 71, 1106–1115. https://doi.org/10.1016/j.jhep.2019.07.020
Degenhardt, L., Peacock, A., Colledge, S., et al., 2017. Global prevalence of injecting drug use and sociodemographic characteristics and prevalence of HIV, HBV, and HCV in people who inject drugs: a multistage systematic review. Lancet Glob. Health 5, e1192–e1207.
Deng, S., Zhong, W., Chen, W., Wang, Z., 2023. Hepatitis C viral load and mother-to-child transmission: A systematic review and meta-analysis. J. Gastroenterol. Hepatol. 38, 177–186.
Deterding, K., Höner Zu Siederdissen, C., Port, K., et al., 2015. Improvement of liver function parameters in advanced HCV-associated liver cirrhosis by IFN-free antiviral therapies. Aliment. Pharmacol. Ther. 42, 889–901.
Dietz, J., Di Maio, V.C., de Salazar, et al. HCV Virology Italian Resistance Network (VIRONET-C) collaborators, Spanish GEHEP-004 Collaborators, Members of the German HCV resistance study group, 2021. Failure on voxilaprevir, velpatasvir, sofosbuvir and efficacy of rescue therapy. J. Hepatol. 74, 801–810.
Dietz J, Graf C, Berg CP et al. Rare HCV subtypes and retreatment outcomes in a cohort of European DAA-experienced patients, JHEP Reports 2024; S2589-5559(24)00076-4
European AIDS Clinical Society. EACS Guidelines 2024. URL: https://www.eacsociety.org/guidelines/eacs-guidelines/ (accessed 12.03.24).
El-Sherif, O., Jiang, Z.G., Tapper, E.B., et al., 2018. Baseline Factors Associated With Improvements in Decompensated Cirrhosis After Direct-Acting Antiviral Therapy for Hepatitis C Virus Infection. Gastroenterology 154, 2111-2121.e8.
Esposto, G., Santini, P., Galasso, L., et al., 2024. Shear-wave elastography to predict hepatocellular carcinoma after hepatitis C virus eradication: A systematic review and meta-analysis. World J. Gastroenterol. 30, 1450–1460.
European Association for the Study of the Liver. 2020. EASL recommendations on treatment of hepatitis C: Final update of the series☆. J. Hepatol. 2020; 73:1170–1218.
Fattovich, G., Giustina, G., Degos, F., et al., 1997. Morbidity and mortality in compensated cirrhosis type C: a retrospective follow-up study of 384 patients. Gastroenterology 112, 463–472.
Feld, J.J., Jacobson, I.M., Hézode, C., et al., ASTRAL-1 Investigators, 2015. Sofosbuvir and Velpatasvir for HCV Genotype 1, 2, 4, 5, and 6 Infection. N. Engl. J. Med. 373, 2599–2607.
Fierer, D.S., Wyles, D.L., 2020. Re-treatment of Hepatitis C Infection After Multiple Failures of Direct-Acting Antiviral Therapy. Open Forum Infect. Dis. 7.
Foster, G.R., Afdhal, N., Roberts, et al., 2015. Sofosbuvir and Velpatasvir for HCV Genotype 2 and 3 Infection. N. Engl. J. Med. 373, 2608–2617.
Freeman, A.J., Dore, G.J., Law, M.G., et al., 2001. Estimating progression to cirrhosis in chronic hepatitis C virus infection. Hepatol. Baltim. Md 34, 809–816.
Frías, M., Rivero-Juárez, A., Téllez, F., et al., 2019. Evaluation of hepatitis C viral RNA persistence in HIV-infected patients with long-term sustained virological response by droplet digital PCR. Sci. Rep. 9, 12507.
AASLD-IDSA Recommendations for Testing, Managing, and Treating Hepatitis C Virus Infection. URL: https://www.hcvguidelines.org/ (accessed 12.04.2024)
Gottwein, J.M., Pham, L.V., Mikkelsen, L.S., et al., 2018. Efficacy of NS5A Inhibitors Against Hepatitis C Virus Genotypes 1-7 and Escape Variants. Gastroenterology 154, 1435–1448.
Gower, E., Estes, C., Blach, S., Razavi-Shearer, K., Razavi, H., 2014. Global epidemiology and genotype distribution of the hepatitis C virus infection. J. Hepatol. 61, S45-57. https://doi.org/10.1016/j.jhep.2014.07.027
Graf, C., D’Ambrosio, R., Degasperi, E., et al., 2024. Real-world effectiveness of voxilaprevir/velpatasvir/sofosbuvir in patients following DAA failure. JHEP Rep. Innov. Hepatol. 6, 100994.
Grebely, J., Page, K., Sacks-Davis, R., et al., InC3 Study Group, 2014. The effects of female sex, viral genotype, and IL28B genotype on spontaneous clearance of acute hepatitis C virus infection. Hepatol. Baltim. Md 59, 109–120.
Hajarizadeh, B., Grady, B., Page, K., et al., 2015. Patterns of Hepatitis C Virus RNA Levels during Acute Infection: The InC3 Study. PLOS ONE 10, e0122232.
Philip Spradling (2024): Hepatitis C. In: CDC Yellow Book 2024. URL: https://wwwnc.cdc.gov/travel/yellowbook/2024/infections-diseases/hepatitis-c (accessed 4.1.24).
Indolfi, G., Easterbrook, P., Dusheiko, G., et al., 2019. Hepatitis C virus infection in children and adolescents. Lancet Gastroenterol. Hepatol. 4, 477–487.
Ingiliz, P., Martin, T.C., Rodger, A., et al., 2017. HCV reinfection incidence and spontaneous clearance rates in HIV-positive men who have sex with men in Western Europe. J. Hepatol. 66, 282–287.
Jacobson, I.M., Lawitz, E., Kwo, P.Y., et al., 2017. Safety and Efficacy of Elbasvir/Grazoprevir in Patients With Hepatitis C Virus Infection and Compensated Cirrhosis: An Integrated Analysis. Gastroenterology 152, 1372-1382.e2.
Jin, F., Prestage, G.P., Matthews, G., et al., 2010. Prevalence, incidence and risk factors for hepatitis C in homosexual men: data from two cohorts of HIV-negative and HIV-positive men in Sydney, Australia. Sex. Transm. Infect. 86, 25–28.
Jonas, M., Romero, R., Sokal, E.M., et al., 2019. Safety and efficacy of sofosbuvir/velpatasvir in pediatric patients 6 to <18 years old with chronic hepatitis C infection. Hepatology (Suppl) 2019, 465A
Jonas, M.M., Squires, R.H., Rhee, S.M., et al., 2020. Pharmacokinetics, Safety, and Efficacy of Glecaprevir/Pibrentasvir in Adolescents With Chronic Hepatitis C Virus: Part 1 of the DORA Study. Hepatol. Baltim. Md 71, 456–462.
Kushner, T., Djerboua, M., Biondi, M.J., Feld, J.J., Terrault, N., Flemming, J.A., 2022. Influence of hepatitis C viral parameters on pregnancy complications and risk of mother-to-child transmission. J. Hepatol. 77, 1256–1264.
Kwo, P.Y., Poordad, F., Asatryan, A., et al., 2017. Glecaprevir and pibrentasvir yield high response rates in patients with HCV genotype 1-6 without cirrhosis. J. Hepatol. 67, 263–271.
Lauer, G.M., Walker, B.D., 2001. Hepatitis C virus infection. N. Engl. J. Med. 345, 41–52.
Liang, T.J., Ward, J.W., 2018. Hepatitis C in Injection-Drug Users - A Hidden Danger of the Opioid Epidemic. N. Engl. J. Med. 378, 1169–1171.
Llaneras, J., Riveiro-Barciela, M., Lens, S., et al., 2019. Effectiveness and safety of sofosbuvir/velpatasvir/voxilaprevir in patients with chronic hepatitis C previously treated with DAAs. J. Hepatol. 71, 666–672.
Lu, M., Wu, K.-H., Li, J., et al., 2019. Adjuvant ribavirin and longer direct-acting antiviral treatment duration improve sustained virological response among hepatitis C patients at risk of treatment failure. J. Viral Hepat. 26, 1210–1217.
Mandorfer, M., Kozbial, K., Schwabl, P., et al., 2016. Sustained virologic response to interferon-free therapies ameliorates HCV-induced portal hypertension. J. Hepatol. 65, 692–699.
Martin, M.T., Patel, S., Kulik, L., Chan, C., 2021. Glecaprevir/pibrentasvir + sofosbuvir + ribavirin offers high cure rate for hepatitis C virus retreatment in real-world settings. J. Hepatol. 75, 251–254.
Martinello, M., Hajarizadeh, B., Grebely, J., Dore, G.J., Matthews, G.V., 2018. Management of acute HCV infection in the era of direct-acting antiviral therapy. Nat. Rev. Gastroenterol. Hepatol. 15, 412–424.
Messina, J.P., Humphreys, I., Flaxman, A., et al., 2015. Global distribution and prevalence of hepatitis C virus genotypes. Hepatol. Baltim. Md 61, 77–87.
Micallef, J.M., Kaldor, J.M., Dore, G.J., 2006. Spontaneous viral clearance following acute hepatitis C infection: a systematic review of longitudinal studies. J. Viral Hepat. 13, 34–41.
Morris, M.D., Shiboski, S., Bruneau, J., et al., International Collaboration of Incident HIV and HCV in Injecting Cohorts (InC3), 2017. Geographic Differences in Temporal Incidence Trends of Hepatitis C Virus Infection Among People Who Inject Drugs: The InC3 Collaboration. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 64, 860–869.
Murray, K.F., Balistreri, W.F., Bansal, S., et al., 2018. Safety and Efficacy of Ledipasvir-Sofosbuvir With or Without Ribavirin for Chronic Hepatitis C in Children Ages 6-11. Hepatol. Baltim. Md 68, 2158–2166.
Nahon, P., Bourcier, V., Layese, R., Aet al., ANRS CO12 CirVir Group, 2017. Eradication of Hepatitis C Virus Infection in Patients With Cirrhosis Reduces Risk of Liver and Non-Liver Complications. Gastroenterology 152, 142-156.e2.
Netski, D.M., Mosbruger, T., Depla, E., et al., 2005. Humoral Immune Response in Acute Hepatitis C Virus Infection. Clin. Infect. Dis. 41, 667–675.
Newsum, A.M., Matser, A., Schinkel, J., et al., MSM Observational Study of Acute Infection with hepatitis C (MOSAIC) study group, 2021. Incidence of HCV Reinfection Among HIV-Positive MSM and Its Association With Sexual Risk Behavior: A Longitudinal Analysis. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 73, 460–467.
Ohto, H., Terazawa, S., Sasaki, N., et al., 1994. Transmission of hepatitis C virus from mothers to infants. The Vertical Transmission of Hepatitis C Virus Collaborative Study Group. N. Engl. J. Med. 330, 744–750.
Pawlotsky, J.-M., Negro, F., Aghemo, A., et al., 2020. EASL recommendations on treatment of hepatitis C: Final update of the series. J. Hepatol. 73, 1170–1218.
Petruzziello, A., Marigliano, S., Loquercio, G., Cozzolino, A., Cacciapuoti, C., 2016. Global epidemiology of hepatitis C virus infection: An up-date of the distribution and circulation of hepatitis C virus genotypes. World J. Gastroenterol. 22, 7824–7840.
Polaris Observatory HCV Collaborators, 2022. Global change in hepatitis C virus prevalence and cascade of care between 2015 and 2020: a modelling study. Lancet Gastroenterol. Hepatol. 7, 396–415.
Polaris Observatory HCV Collaborators, 2017. Global prevalence and genotype distribution of hepatitis C virus infection in 2015: a modelling study. Lancet Gastroenterol. Hepatol. 2, 161–176.
Powdrill, M.H., Bernatchez, J.A., Götte, M., 2010. Inhibitors of the Hepatitis C Virus RNA-Dependent RNA Polymerase NS5B. Viruses 2, 2169–2195.
Rauch, A., Kutalik, Z., Descombes, P., et al., 2010. Genetic variation in IL28B is associated with chronic hepatitis C and treatment failure: a genome-wide association study. Gastroenterology 138, 1338–1345, 1345.e1–7.
Samur, S., Kues, B., Ayer, T.,et al., 2018. Cost Effectiveness of Pre- vs Post-Liver Transplant Hepatitis C Treatment With Direct-Acting Antivirals. Clin. Gastroenterol. Hepatol. Off. Clin. Pract. J. Am. Gastroenterol. Assoc. 16, 115-122.e10.
Sarrazin, C., Isakov, V., Svarovskaia, E.S., et al., 2017. Late Relapse Versus Hepatitis C Virus Reinfection in Patients With Sustained Virologic Response After Sofosbuvir-Based Therapies. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 64, 44–52.
Schwarz, K.B., Rosenthal, P., Murray, K.F., et al., 2020. Ledipasvir-Sofosbuvir for 12 Weeks in Children 3 to <6 Years Old With Chronic Hepatitis C. Hepatol. Baltim. Md 71, 422–
Semmler, G., Alonso López, S., Pons, M., et al., cACLD-SVR Study Group, 2024. Post-treatment LSM rather than change during treatment predicts decompensation in patients with cACLD after HCV cure. J. Hepatol. S0168-8278(24)00198–3.
Shin, E.-C., Sung, P.S., Park, S.-H., 2016. Immune responses and immunopathology in acute and chronic viral hepatitis. Nat. Rev. Immunol. 16, 509–523.
Sulkowski M. S., Gardiner D. F., Rodriguez-Torres M., et al., 2014. Daclatasvir plus Sofosbuvir for Previously Treated or Untreated Chronic HCV Infection. N. Engl. J. Med. 370, 211–221.
Terrault, N.A., Dodge, J.L., Murphy, E.L., et al., 2013. Sexual transmission of hepatitis C virus among monogamous heterosexual couples: the HCV partners study. Hepatol. Baltim. Md 57, 881–889.
Terrault, N.A., Levy, M.T., Cheung, K.W., Jourdain, G., 2021. Viral hepatitis and pregnancy.Nat. Rev. Gastroenterol. Hepatol. 18, 117–130.
Thein, H.-H., Yi, Q., Dore, G.J., Krahn, M.D., 2008. Estimation of stage-specific fibrosis progression rates in chronic hepatitis C virus infection: a meta-analysis and meta-regression. Hepatol. Baltim. Md 48, 418–431.
Thomas, D.L., Thio, C.L., Martin, M.P., et al., 2009. Genetic variation in IL28B and spontaneous clearance of hepatitis C virus. Nature 461, 798–801.
Trickey, A., Fraser, H., Lim, A.G., et al., 2019. The contribution of injection drug use to hepatitis C virus transmission globally, regionally, and at country level: a modelling study. Lancet Gastroenterol. Hepatol. 4, 435–444.
van der Meer, A.J., Veldt, B.J., Feld, J.J., et al., 2012. Association between sustained virological response and all-cause mortality among patients with chronic hepatitis C and advanced hepatic fibrosis. JAMA 308, 2584–2593.
Vermehren, J., Serfert, Y., Cornberg, M., et al., 2020. Sofosbuvir, velpatasvir, and voxilaprevir for patients with failure of previous direct-acting antiviral therapy for chronic hepatitis C: Results from the German Hepatitis C-Registry (DHC-R). Z. Gastroenterol. 58, 841–846.
Villanueva, A., 2019. Hepatocellular Carcinoma. N. Engl. J. Med. 380, 1450–1462.
Vogel, M., Deterding, K., Wiegand, J., et al., German Hepatitis Group, Hep-Net, 2009. Initial presentation of acute hepatitis C virus (HCV) infection among HIV-negative and HIV-positive individuals-experience from 2 large German networks on the study of acute HCV infection. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 49, 317–319; author reply 319.
Wei, L., Lim, S.G., Xie, Q., et al., 2019. Sofosbuvir-velpatasvir for treatment of chronic hepatitis C virus infection in Asia: a single-arm, open-label, phase 3 trial. Lancet Gastroenterol. Hepatol. 4, 127–134.
Wei, L., Wang, G., Alami, N.N., et al., 2020. Glecaprevir–pibrentasvir to treat chronic hepatitis C virus infection in Asia: two multicentre, phase 3 studies—a randomised, double-blind study (VOYAGE-1) and an open-label, single-arm study (VOYAGE-2). Lancet Gastroenterol. Hepatol. 5, 839–849.
Wyles, D., Bräu, N., Kottilil, S., et al., ASTRAL-5 Investigators, 2017. Sofosbuvir and Velpatasvir for the Treatment of Hepatitis C Virus in Patients Coinfected With Human Immunodeficiency Virus Type 1: An Open-Label, Phase 3 Study. Clin. Infect. Dis. Off. Publ. Infect. Dis. Soc. Am. 65, 6–12.
Yeung, C.-Y., Lee, H.-C., Chan, W.-T., Jiang, C.-B., Chang, S.-W., Chuang, C.-K., 2014. Vertical transmission of hepatitis C virus: Current knowledge and perspectives. World J. Hepatol. 6, 643–651.
Zeng, H., Li, L., Hou, Z., Zhang, Y., Tang, Z., Liu, S., 2020. Direct-acting Antiviral in the Treatment of Chronic Hepatitis C: Bonuses and Challenges. Int. J. Med. Sci. 17, 892–902.
Zeuzem, S., Foster, G.R., Wang, S., et al., 2018. Glecaprevir–Pibrentasvir for 8 or 12 Weeks in HCV Genotype 1 or 3 Infection. N. Engl. J. Med. 378, 354–369.
Zhang, Y., Chen, L.-M., He, M., 2017. Hepatitis C Virus in mainland China with an emphasis on genotype and subtype distribution. Virol. J. 14, 41.
Zhang, Y.-Q., Guo, J.-S., 2015. Antiviral therapies for hepatitis B virus-related hepatocellular carcinoma. World J. Gastroenterol. 21, 3860–3866.
Zignego, A.L., Craxì, A., 2008. Extrahepatic manifestations of hepatitis C virus infection. Clin. Liver Dis. 12, 611–636.
