14. Alcohol-associated hepatitis
Julian Allgeier, Claus Niederau, Johannes Sauter, Christian M. Lange
Harmful alcohol use and its global burden
Alcohol consumption remains one of the leading risk factors for disease worldwide. In 2018, the Global Burden of Disease collaboration considered 32, 5% of people (25% of females, 39% of males) to be current drinkers, with prevalence highest in regions with high socio-demographic index (SDI) like north western Europe, Australia, Russia or Canada (Collaborators 2018). In 2016, alcohol use was the seventh leading risk factor for premature death and disability and accounted for 2, 8 million deaths (Collaborators 2018). Europe has the highest attributable burden of all WHO regions (Collaborators 2018) with a significant gender gap: the attributable burden in men is 11, 0% whereas it is only 1, 8% in women (Collaborators 2018). While there is some evidence that moderate alcohol consumption may have protective effects in disease entities including ischemic heart disease and diabetes, the all-cause relative mortality risk increased monotonically with the amount of daily alcohol drinks (Collaborators 2018). Excessive alcohol consumption, whether in the form of heavy drinking or binge drinking, is responsible for about 50% of all liver-related deaths (Stein 2016); alcohol-associated liver disease culminates in cirrhosis in about 10-20% of cases in the United States (Singal 2021).
The onset of the COVID-19 pandemic aggravated these alcohol-associated effects; sale of alcoholic beverages increased significantly both remotely and online in 2020, the first year of the pandemic (Grossman 2020). Microsimulation modeling has estimated that this one-year increase will have resulted in 8000 ALD-related deaths, 18.700 cases of decompensated cirrhosis, 1.000 additional cases of HCC and 8, 9 million disability-adjusted life years by 2080 (Julien 2022). In the U.S. mortality rates for alcoholic liver disease dramatically increased between 2010-2019 and 2020-2021 during the COVID-19 pandemic, while the rates for NAFLD slightly increased and those for hepatitis B and C decreased (Gao 2023); the later study also showed that alcoholic liver disease was the most common cause of liver related mortality in the U.S. accounting for 55% of deaths, followed by HCV (33%), NAFLD (9%), and HBV (3%).
Definitions for what is considered hazardous alcohol use differ; the National Institute on Alcohol Use and Alcoholism (NIAAA) considers non-hazardous alcohol consumption as less than one drink for women and less than two drinks for men per day, with one drink defined as 14 g of alcohol (2018). Yet, there is no threshold of safe alcohol consumption and even lower amounts confer a risk of alcohol-associated liver disease in the long-term (Collaborators 2022). Moreover, various drinking patterns confer an increased risk for liver injury, specifically binge drinking, more than four or five drinks for women and men, respectively, in less than two hours, and heavy drinking, more than seven or fourteen drinks for women and men, respectively, in a week (NIAAA 2018).
Key aspect to all strategies to reduce alcohol-related morbidity is prevention. A Swedish register study tracking more than 43.000 men enlisted for military service between 1969 and 1970 for 38 years found found that alcohol consumption in adolescence predicts liver related morbidity significantly later in life in a dose-dependent manner even at doses below those defined by various agencies as non-hazardous (Hagstrom 2018). Thus, education about the effects of alcohol consumption on somatic and psychological health needs initiation early in life. There is evidence that this should include brief behavioral counseling interventions to reduce unhealthy alcohol use in adults >18 years (O'Connor 2018) while evidence for those aged 17 and under is still lacking. To identify those at risk for alcohol use disorder (AUD), any drinking that results in impairment of mental of physical health, screening tools and questionnaires are helpful. The alcohol use disorders identification test (AUDIT) remains the gold standard for identifying hazardous and harmful drinkers (Bohn 1995), but a variety of other screening and assessment tools have arisen and are widely available online and through various agencies. Regulating availability of alcohol by amending legal drinking age, restricting access through reducing places of sale, higher taxes or bans on advertising might all positively impact alcohol-related morbidity, but more research is warranted to assess their individual and collective impact on harmful alcohol consumption.
Pathogenesis of alcohol-related liver disease (ALD)
There is significant individual variability in the relationship between extent of alcohol consumption and onset and severity of ALD. Predisposition to ALD is mediated by environmental, genetic and epigenetic factors; for the development of more severe forms such as alcoholic hepatitis-associated liver failure, conclusive causative scientific evidence is still lacking (Bataller 2022).
Hepatic injury in the setting of excessive alcohol consumption is a consequence of direct toxicity of ethanol-metabolites, but also of intestinal dysbiosis, damage of intestinal barriers and local and systemic inflammatory responses.
(1) Direct hepatic injury mediated by ethanol
Ethanol is oxidised by various enzymatic and nonenzymatic pathways. In hepatocytes, the most important pathway is oxidation of ethanol via alcohol dehydrogenase (ADH) to acetaldehyde; in mitochondria, acetaldehyde is converted to acetate and in turn acetate is converted to acetyl CoA, which leads the two-carbon molecule into the TCA (tricarboxylic acid cycle). The human genome encodes for five different classes of ADH, the majority of which are found in hepatic tissue (Sultatos 2004); however, alcohol metabolism mediated by ADH initiates not there but in the gastric epithelium (Sultatos 2004). Ethanol oxidation generates reducing equivalents, primarily reduced nicotinamide adenine dinucleotide (NAD), i.e., NADH. Changes in the NADH–NAD+ potential in the liver inhibit both fatty acid oxidation and the TAC and may thereby increase lipogenesis (You 2004). Ethanol has also been proven to increase lipid metabolism by inhibiting peroxisome proliferator–activated receptor α (PPARα) and AMP kinase as well as by stimulation of sterol regulatory element-binding protein (Fischer 2003, Ji 2006, You 2004), all mechanisms that favour presence of hepatic steatosis.
Ethanol may also activate Fas and TNF receptor 1 (TNF-R1) thereby activating caspase 8, causing mitochondrial injury and opening the mitochondrial transition pore (MTP), releasing cytochrome c, and activating caspases; all these processes contribute to apoptosis. Activation of TNF-R1 leads to nuclear factor kappa B (NFkB) activation (Schaffert 2009). TRIF-dependent signaling may contribute to alcohol-induced liver damage mediated by TLR4 (Hritz 2008). Animal models have also shown that alcohol increases various markers of oxidative stress (Meagher 1999, Wu 2009). Studies in rats and mice suggest that activated Kupffer cells and hepatocytes are the main sources of alcohol-induced free radicals (Bailey 1998, Kamimura 1992). Oxidative stress may mediate alcohol-induced liver injury in part via cytochrome P450 2E1 (Pessayre 1999, Lu 2008), leading to mitochondrial damage, activation of endoplasmic reticulum–dependent apoptosis, and up-regulation of lipid synthesis (Ji 2003, Yin 2001).
Other enzymatic pathways involved in ethanol metabolism include catalase and the Microsomal Ethanol Oxidising System (MEOS), a distinct structural unit of the endoplasmatic reticulum involved in metabolism of ethanol to acetaldehyde and utilising reduced NADH generated by cytosolic ADH activity (Teschke 2018).
The major propagating factor of alcohol-induced liver damage is formation of reactive oxygen species (ROS) and their consecutive direct damage to cellular and subcellular structures resulting in hepatocyte death and an inflammatory response by the host; virtually all systems, also those not explicitly named here, cause formation of ROS (Wu 2003).
(2) The gut-liver axis
Ethanol directly and indirectly affects the intestinal epithelial barrier and as such, the translocation of intraluminal contents to the portal venous system and by extension to the liver (Szabo 2015). High concentrations of intraluminal ethanol cause cell death in intestinal epithelium; systemic ethanol also downregulates mRNA levels of proteins associated with function and integrity of tight junctions in intestinal epithelium, resulting in impaired intestinal barrier function (Keshavarzian 1999). Acetaldehyde exhibits similar intraluminal effects (Dunagan 2012). Of note, ethanol consumption leads to profound intestinal dysbiosis, characterised by intestinal bacterial overgrowth, enrichment of pathogenic bacterial species, and of species more characteristic for the oral microbiome (Bajaj 2019). These changes are accompanied by changes in the intestinal virome and by fungal dysbiosis in alcoholic hepatitis (Lang 2020, Jiang 2020). Importantly, intestinal dysbiosis induces intestinal inflammation (mediated among others by TNF-alpha), which increases intestinal permeability (Chen 2015). Furthermore, a milestone study has shown that cytolysin, secreted by Enterococcus faecalis can cause hepatocyte death and liver injury (Duan 2019). This finding is particularly important since cytolytic Enterococcus faecalis can be therapeutically targeted by bacteriophages.
These changes in barrier integrity result in translocation of inflammatory mediators to the hepatic circulation, including pathogen-associated molecular patterns (PAMP) and damage-associated molecular patterns (DAMP) like lipopolysaccharide (LPS), the prototypical bacterial endotoxin (Szabo 2015), in most cases independent of consumption pattern (Bala 2014). Binding of LPS to CD14 in Kupffer cells and activation of toll-like receptors, specifically toll-like-receptor-4 (TLR-4) propagates inflammatory cascades in the liver (Schaffert 2009). Inhibition of this pathway in mice deficient of either TLR4 or CD14 has mediated protection to the detrimental hepatic effects of alcohol (Hritz 2008, Uesugi 2001, Petrasek 2010). Activation of TLR4 additionally results in downstream activation of the NFK-β pathway and increased production of a variety of pro- and anti-inflammatory cytokines including TNF-α and IL-1b, two molecules also involved in increased gut permeability, constituting a feedback-loop for further aggravation of alcohol-induced translocation of intestinal contents (Yoseph 2013).
Historically, the role of TNF-α has attracted significant attention as a potential treatment target in alcoholic hepatitis, but this approach has failed in clinical studies. Activated Kupffer cells also release TNF-α. Circulating TNF-α concentrations are higher in patients with alcoholic hepatitis than in heavy drinkers with inactive cirrhosis, heavy drinkers who do not have liver disease and people who do not drink alcohol and who do not have liver disease (Adachi 1994, Bird 1990). Circulating TNF-α concentrations are associated with high mortality (Bird 1990). In animal studies, knockouts of the TNF receptor 1 and administration of the anti-TNF-α agent thalidomide both ameliorated alcohol-induced liver injury (Yin 1999, Enomoto 1999). Ethanol was also shown to release mitochondrial cytochrome c and to induce expression of the Fas ligand that may then cause apoptosis via the caspase-3 activation pathway (Zhou 2001). Both TNF-α and Fas-mediated signals may increase the vulnerability of hepatocytes (Minagawa 2004).
(3) Genetic factors
While much remains unknown about the genetic thumbprint that predisposes to the development or progression of ALD in individuals with risky alcohol use, some risk variations have been identified. There is ample evidence that women develop ALD more quickly than men (Becker 1996, Sato 2001). In 2008, two genome-wide association (GWAS) studies linked the rs738409 polymorphism (I148M) of patatin-like phospholipase domain containing 3 (PNPLA3) with hepatic fat content and ALT levels (Romeo 2008, Yuan 2008). Further studies corroborated this association between the I148M polymorphism and NAFLD in almost all ethnic and age groups (Baclig 2014, DiStefano 2015, Buch 2015, Trepo 2014). The I148M polymorphism also seems to predispose to cirrhosis (Shen 2015) and hepatocellular carcinoma (Trepo 2014, Burza 2014, Valenti 2013). More recently, it has been suggested that the IL48M PNPLA3 polymorphism also accelerates fibrosis progression and HCC incidence in alcoholic liver disease (Buch 2015, Trepo 2012, Nault 2014, Falleti 2016, Stickel 2015). Another GWAS confirmed PNPLA3 and identified TM6SF2 and MBOAT7 as risk loci for alcohol-related cirrhosis (Buch 2015). All three loci are known to have a role in lipid processing, suggesting that lipid turnover is important in the pathogenesis of alcohol-related cirrhosis. An investigation into the interaction between PNPLA3 rs738409 and TM6SF2 rs58542926 as risk variants for HCC development showed that TM6SF2 C/T or T/T in conjunction with PNPLA3 G/G variants may be potential genetic risk factors for developing HCC in alcohol-related cirrhosis (Falleti 2016). In addition, carriage of the heterozygous alpha1-antitrypsin Pi*Z strongly increases the risk of alcohol-associated liver disease (Strnad 2019).
 Figure 1. Effects of alcohol overconsumption on the liver
Figure 1. Effects of alcohol overconsumption on the liver
Diagnosis
The clinical spectrum of alcohol-associated liver disease ranges from steatosis and steatohepatitis to severe alcoholic hepatitis, liver cirrhosis and hepatocellular carcinoma (Figure 1). Severe alcoholic hepatitis is a syndrome that can emerge in patients with or without liver cirrhosis. Common features include jaundice, ascites, peripheral edema and hepatomegaly; in the presence of portal hypertension and associated sequelae, patients can also present with hematemesis or other signs of gastrointestinal hemorrhage. Hepatic encephalopathy (HE) is common, but caution is warranted as alcohol withdrawal syndrome represents an important differential diagnosis and differs in treatment. Overt HE is associated with poorer prognosis (Sujan 2018). In patients with liver cirrhosis, alcoholic hepatitis is a frequent cause of acute-on-chronic liver failure (i.e. acute decompensation of cirrhosis in combination with specific organ failures).
While the diagnosis of alcohol-associated hepatitis remains a clinical one, a variety of criteria have been recommended over time, most recently by the government-funded Alcoholic Hepatitis Consortia. The following conditions are employed (Crabb 2016):
Criteria for diagnosis of alcoholic hepatitis
- Onset of Jaundice within eight weeks of presentation to medical professional
- Ongoing excessive alcohol consumption
- Females: three drinks or > 40 g alcohol/diem
- Males: four drinks or > 50 g alcohol/diem
- Abstinence (if so reported) of less than 60 days
- Total Bilirubin >3 mg/dL
- Aspartate Aminotransferase (AST) >50 U/L + AST/ALT ratio of 1.5 AND both AST and ALST < 400 U/L
- Exclusion of other causes of acute liver injury
A major adjustment in comparison to previous sets of criteria has been inclusion of moderate cases of alcohol-associated hepatitis as evidenced by lowering the threshold of inclusion of total bilirubin from 5 mg/dL to 3 mg/dL (Bataller 2022). Seemingly insignificant, this enables inclusion of a subgroup of alcoholic hepatitis cases that has significant short and medium term mortality (<7% at three months and <20% at one year) (Clemente-Sanchez 2021) and would benefit from medical surveillance primarily aimed at promoting cessation of alcohol consumption (Bataller 2022).
Ruling out competing differential diagnoses should include exclusion of biliary or vessel obstruction via imaging (ultrasound, CT/MRT), viral hepatitis (HAV/HBV/HCV/HEV and other hepatotropic viruses), autoimmune hepatitis (autoimmune serology), ischaemia and drug-induced liver injury (DILI).
Liver biopsy, preferably transjugular, is not required but has its place in the setting of diagnostic uncertainty. Biopsy can only identify presence of steatohepatitis and while certain characteristics tend to appear more readily in alcoholic hepatitis, is not the appropriate diagnostic modality to differentiate it from nonalcoholic steatohepatitis (Kleiner 2012). Acute steatohepatitis generally features ballooned hepatocytes, presence of Mallory-Denk bodies, a neutrophilic infiltrate, ductular reactions, bilirubinostasis and pericellular and sinusoidal fibrosis (Bataller 2022).
If performed, liver biopsy can deliver important prognostic information. Altamirano et al developed a semiquantitative scoring system called the Alcoholic Hepatitis Histologic-Score (AHHS) (Altamirano 2014). Their primary analysis included data from 121 patients in Barcelona, Spain; its development continued through a test set of 96 patients from five academic centres in the United States and Europe. The system was validated with an independent group of 109 patients. Degree of fibrosis, neutrophil infiltration, type of bilirubinostasis, and presence of megamitochondria were independently associated with 90-day mortality. The AHHS identifies patients with a low (0–3 points), moderate (4–5 points), or high (6–9 points) risk of death within 90 days (3%, 19%, and 51%, respectively; p < 0.0001). It estimated 90-day mortality in the training and test sets with an area under the receiver operating characteristic analysis of 0.77 (95% confidence interval 0.71–0.83), thus proving its potential clinical use for identifying high risk individuals (Altamirano 2014).
Similarly, Lackner et al. recently developed a scoring system under the umbrella of the Study of Alcohol-related Liver Disease Study Group (SALVE); their scoring system, just like the AHHS, clearly demonstrates increased mortality in the setting of present cirrhosis (Lackner 2021).
Predictive modeling and indication for therapy
Disease-related mortality for alcoholic hepatitis varies depending on project (?, was ist mit project gemeint?) and is generally approximated between 20-50% after three months (Cohen 2009, Arab 2021).
Most prominently, Maddrey’s discriminant function (MDF) and the Model for End-Stage Liver Disease (MELD) score are employed for stratification and help to identify patients who can benefit from treatment with corticosteroids. MDF is calculated using the following equation (Maddrey 1978):
MDF >32 indicates benefit from corticosteroid treatment in the setting of alcoholic hepatitis (Maddrey 1978). MELD is useful specifically in clinical settings in which PT is not a parameter routinely determined. Patients with MDF <32 usually have milder disease with short term survival >90% and generally do not benefit from treatment with corticosteroids. In patients with acute-on-chronic liver failure, the CLIF-C ACLF score can be informative (https://www.efclif.com/scientific-activity/score-calculators/clif-c-aclf).
Other less commonly employed scoring systems that have shown ability to predict mortality in the alcoholic hepatitis cohort include the Glasgow Alcoholic Hepatitis Score (GAHS) (Forrest 2007) and the ABIC Score (Age; Bilirubin; INR; Creatinine) (Dominguez 2008). Patients with a Maddrey’s discriminant function >32 and a GAHS of >9 who were treated with corticosteroids had an 84-day survival of 59%, while untreated patients had a 38% survival (Forrest 2007).
The GAHS adjudicates points for the following categories:
GAHS categories
- Age
- White Blood Cell Count (WBC)
- BUN
- Total Bilirubin
- PT
ABIC uses the following equations for calculation:
ABIC = (Age, years, × 0.1) + (Total Bilirubin (mg ⁄ dl) x 0.08)
+ (INR × 0.8) + (Creatinine (mg ⁄ dl) × 0.3)
A variety of retrospective analyses have recently concluded that MELD prognosticates mortality more accurately than MDF, making it the score of choice for approximating usefulness of corticosteroid treatment at the present time (Dunn 2005, Srikureja 2005, Morales-Arraez 2022, Forrest 2018). Maximum benefit from corticosteroids is derived in a MELD range between 25 – 39 (Bataller 2022).
As corticosteroids increase the risk of infection which is one of the major complications contributing to mortality in the presence of alcoholic liver failure, the Lille Score has been useful in predicting lack of response to corticosteroids and is calculated on day 7 after initiation of treatment (Louvet 2007). Calculations can also be performed on day 4 (Garcia-Saenz-de-Sicilia 2017).
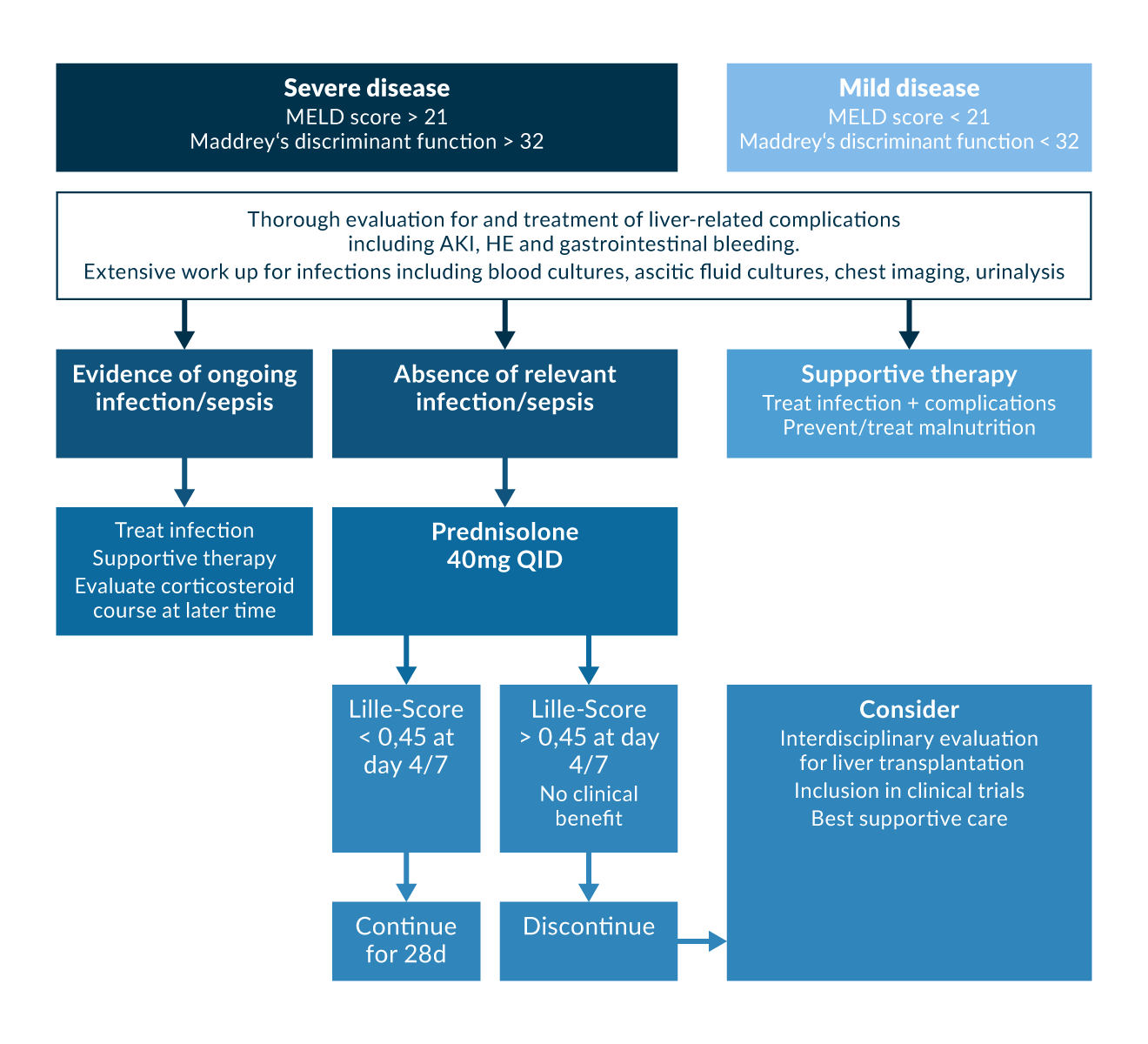 Figure 2.
Figure 2.
Therapy
(1) Corticosteroids
Historically, studies and meta-analyses have shown controversial results for the use of corticosteroids in alcoholic hepatitis (Imperiale 1990, Imperiale 1999, Rambaldi 2008, Christensen 1999). Generally, it is accepted that corticosteroids have not been shown to increase survival, in particular during longer follow-up (Rambaldi 2008) except in a subgroup of patients with a Maddrey’s discriminant function >32 or in those presenting with hepatic encephalopathy (Rambaldi 2008, Mathurin 2002). A meta-analysis of three studies corroborated that corticosteroids given for 28 days increase 1-month survival by 20% in severe alcoholic hepatitis (Maddrey’s discriminant function >32) (Mathurin 2002). Prednisolone was generally administered at 40 mg per day for 28 days, with (Imperiale 1999) or without (Mathurin 2003) tapering regimens employed.
In the STOPAH trial, the largest double-blind RCT comparing prednisolone (and pentoxifylline, details below) conducted thus far, primary end-point analysis of data of 1053 patients yielded a moderate improvement of short-term mortality (1 month) in the prednisolone-group, but no significant differences in 3 month and 1 year mortality (Thursz 2015).
The mechanisms by which corticosteroids improve short-term survival in severe alcoholic hepatitis are not fully understood but are generally thought to be by disruption of inflammatory response. In general, corticosteroids inhibit various inflammatory processes by acting on activator protein 1 and NFkB (Barnes 1997). In patients with alcoholic hepatitis, some studies reported that corticosteroids were associated with a decrease in circulating levels of proinflammatory cytokines such as interleukin-8, TNF-α and others (Taieb 2000, Spahr 2001). Corticosteroid use is considered contraindicated in the presence of sepsis, severe infection, or gastrointestinal bleeding (O'Shea 2006).
MDF, MELD, and Lille Score are used to stratify whether corticosteroid therapy is indicated; its continuation beyond a seven day treatment course is warranted in the setting of alcoholic hepatitis (Maddrey 1978, Louvet 2007, Lucey 2009) with an MDF >32, or a MELD >21 respectively, indicating a short term benefit (Figure 2).
(2) Pentoxifylline
Pentoxifylline, a diphosphoesterase inhibitor, has been assessed in several clinical trials in severe alcoholic hepatitis.
Initial studies indicated a possible benefit as pentoxifylline (administered at 400 mg TID for 28 days) reduced short-term mortality in severe alcoholic hepatitis (MDF>32); mortality was 24% in the pentoxifylline group and 46% in the placebo group (p <0.01) (Akriviadis 2000); this effect is likely due to significant differences in deaths attributed to hepatorenal syndrome (HRS) (placebo: 22/24, 92%; treatment group: 6/12, 50%), suggesting that its effect is orchestrated through preventing HRS.
In 2014 however, a randomised, non-inferiority trial that included 121 patients with severe alcoholic hepatitis with MDF >32 found no significant differences in 1-month survival, 6-month survival, treatment response as defined by the Lille Model and hepatic complications, concluding that prednisolone remains the preferred treatment option (Park 2014).
Salvage therapy with pentoxifylline after lack of response to prednisolone hads no beneficial effects on treatment outcome (Louvet 2008); similarly, combination of prednisolone and pentoxifylline did not have an effect on 1-month and 6-month survival (Sidhu 2012, Mathurin 2013).
Finally, in the above mentioned STOPAH trial no benefit of pentoxifylline was observed (Thursz 2015). Overall, Pentoxifylline cannot currently be recommended for the treatment of alcoholic hepatitis.
(3) TNF-α inhibition
While initial pre-clinical data and experience from pilot studies concerning the effectiveness of TNF-α inhibition to alleviate hepatic necrosis seemed promising (Tilg 2003, Menon 2004, Sharma 2009, Limuro 1997), trials investigating the use of infliximab and etanercept in severe alcoholic hepatitis had to be terminated prematurely due to a significant increase in severe infections (Naveau 2004) and a decrease in 6-month survival (Boetticher 2008), perhaps because TNF-α is required for liver regeneration. Current guidelines do not recommend their use in severe alcoholic hepatitis (Singal 2018, EASL 2012).
(4) N-acetyl cysteine (NAC) and other antioxidants
In a large trial, the combination of NAC and prednisolone for severe alcoholic hepatitis showed only significant reductions of mortality at the 1-month interval (n=7/85 (8.2%) vs. 21/89 (23.6%), p=0.005) and after two months (13/85 (15.3%) vs. 29/89 (32.6%), p=0.007) but not at three or six months (19/85 (22.4%) vs. 30/89 (33.7%), p=0.095) (23/85 (27.1%) vs. 34/89 (38.2%) (Nguyen-Khac 2011). Nevertheless, further studies are justified to explore the benefits of NAC in addition to prednisolone in severe alcoholic hepatitis, because this trial may have been underpowered.
Other antioxidant drugs, such as vitamin E, have shown to be ineffective at improving outcome or survival for patients with severe alcoholic hepatitis (Phillips 2006, Stewart 2007, Mezey 2004)
(5) G-CSF
In 2014, a RCT evaluated the hypothesis that treating patients with severe alcoholic hepatitis with granulocyte colony-stimulating factor (G-CSF) might mobilise bone marrow-derived stem cells and promote hepatic regeneration and thereby improve survival (Singh 2014). 46 patients were randomised to one treatment arm receiving standard medical treatment (SMT) (n=23) and another arm receiving G-CSF (n=23) at a dose of 5 µg/kg subcutaneously every 12 h for 5 consecutive days.
There was a statistically significant increase in the number of CD34+ cells in the peripheral blood in the G-CSF arm as compared with the SMT arm after 5 days of therapy. Concurrently, 1-month survival was significantly improved in the G-CSF arm (78.3% vs. 30.4%, p=0.001). There was also a significant reduction in Child-Pugh and MELD scores and MDF at 1-, 2-, and 3-month intervals between the groups favouring G-CSF (Singh 2014).
Unfortunately, a recent meta-analysis that included more recent follow-up studies has reported high heterogeneity between studies with geographical differences indicating results ranging from lack of efficiency to even higher mortality with G-CSF in European studies, indicating the need for further, high-quality evidence (Marot 2020).
(6) Nutrition and supportive therapy
Signs of malnutrition and clinically apparent sarcopenia are common in patients with AUD and alcoholic liver disease, underscoring the importance of including nutritional strategies in the treatment approach for this patient collective. Alcoholic beverages have high caloric but poor nutritional value. Malnutrition is associated with high mortality in severe alcoholic hepatitis (Mendenhall 1984, Mendenhall 1986, Stickel 2003).
An RCT compared enteral nutrition with 2000 kcal/day via feeding tube with corticosteroid treatment (Prednisolone 40 mg QD, 28 days) in severe alcoholic hepatitis, finding similar 1-month and 1-year survival rates in both groups (Cabre 2000). A small pilot study in 2004 combined corticosteroid therapy with total enteral nutrition and suggested it could be a useful strategy in patients with severe alcoholic hepatitis (Alvarez 2004).
More recently, the combination of corticosteroid therapy and enteral nutrition was compared against corticosteroid therapy alone in a RCT, enrolling 136 patients with a history of risky alcohol consumption, recent onset of jaundice, and steatohepatitis proven by biopsy; they were assigned randomly (1:1) to groups that received either intensive enteral nutrition plus methylprednisolone or conventional nutrition plus methylprednisolone (Moreno 2016). In the intensive enteral nutrition group, enteral nutrition was given via feeding tube for 14 days. The primary end point was 6-month survival. In the intention-to-treat analysis, there was no significant difference between groups in 6-month cumulative mortality: 44.4% in the enteral nutrition group vs. 52.1% in the controls (p= 0.406). Intensive enteral nutrition was difficult to implement and did not improve survival (Moreno 2016) However, further analysis showed that low daily energy intake was associated with greater mortality, so adequate nutritional intake should remain a goal for treatment.
Current guidelines recommend a daily protein intake of 1.2 – 1.5 g/kg and a daily caloric intake of 35 kcal/kg for patients with severe alcoholic hepatitis with an additional replenishment of thiamine and B complex vitamins as well as zinc and other trace elements (Singal 2018).
Furthermore, a comprehensive infection work-up should be performed to rule out concomitant infection, a major source of decompensation in the setting of alcoholic liver disease. Special attention must be given to differentiating community-acquired from healthcare-associated infections and even without culture positive infection, the threshold for initiating broad spectrum anti-infective therapy should be low (Singal 2018).
(7) Liver transplantation (LT)
In some countries (e.g. in Germany) in patients with decompensated alcoholic cirrhosis, a minimum sobriety interval of six months is required for consideration for liver transplantation (though exceptions are possible). This is, however, a requirement that cannot be afforded to patients in acute liver failure secondary to alcoholic hepatitis, given that the 1-month mortality lies between 20-50% (Singal 2014) and promising conservative treatment options offering improvement are lacking.
In 2011, a pivotal study from Belgium and France showed for the first time that transplanting highly selected patients with severe alcoholic hepatitis that were non-responders to corticosteroid therapy and had a favourable psychosocial profile can strongly improve survival compared to conservative treatment (6-month survival in LT: 77% vs. SMT: 23%, p<0.001) (Mathurin 2011). Relapse rate was low and comparable to historical cohorts (Mathurin 2011). The ACCELERATE-AH study confirmed these results, with survival rates of 94% after one year and 84% after three years; relapse rate for sustained alcohol abuse was 10% and 17% after one and three years, respectively, comparable to those seen after posttransplant for in other transplant indications (Lee 2018). Other cohorts have shown higher rates of relapse in these early transplantation cohorts compared to the traditional approach however (Bataller 2022), and its association with increased mortality warrants further attention. For this purpose, the Sustained Alcohol Use Post Liver Transplant (SALT) Score has been developed and has shown usefulness in predicting low risk for relapse (Lee 2019). It includes four variables: >10 drinks/day at time of initial hospitalisation, multiple previous rehabilitation attempts, alcohol-related legal issues, and illicit substance abuse (Lee 2019).
Cohorts in the United States and in Italy have confirmed that LT significantly improves the outcome of patients with severe alcoholic hepatitis while also reporting similar relapse rates of alcohol consumption (Louvet 2022, Germani 2022). Nevertheless, LT should be considered as the first line treatment in the setting of severe, non-steroid responsive alcoholic hepatitis in highly selected patients after careful evaluation of their psychosocial profile.
(8) Miscellaneous and emerging therapy options
Historically, drugs that target the liver’s capacity to regenerate such as oxandrolone, propylthiouracil, insulin and glucagon have failed to provide a mortality benefit (Halle 1982, Trinchet 1992, Bird 1991).
More recently, the IL-22 agonist F-652 has shown promising ability to reduce MELD and Lille-scores and ameliorate hepatic inflammation after 28 days (Arab 2020) (Table 1). Exploiting the increasingly attention-seizing connection between the liver and the gut microbiome, a pilot study on fecal microbiota transplantation has reported a reduction in mortality compared to historical cohorts (Philips 2017). Another phase 1 clinical trial evaluated effects of FMT in patients with alcoholic cirrhosis and continuous, problematic drinking habits evidenced by AUDIT-10 >8 and reported safety of FMT and also significant reduction in craving; FMT increased microbial diversity and significantly reduced inflammatory parameters such as IL-6 compared to the placebo group (Bajaj 2021).
Other modalities targeting the dysfunction of the gut-liver axis currently under investigation for severe alcoholic hepatitis include broad-spectrum antibiotics and bovine colostrum (Bataller 2022).
Anakinra, in combination with pentoxifylline and zinc has not proven to increase 6-month survival in patients with severe alcoholic hepatitis compared to standard corticosteroid therapy (Szabo 2022), and Anakinra without pentoxifylline and zinc was associated with increased mortality compared to prednisolone in another trial (Gawrieh S. et al. AASLD 2022 late-breaking abstract).
The ISAIAH trial is currently investigating the proficiency of the IL-1 Antagonist canakinumab in severe alcoholic hepatitis (Vergis 2021). The apoptosis inhibitors selonsertib and emricasan are also being evaluated as treatment options (Bataller 2022).
Table 1.| Emerging therapies + ongoing trials | |
| Therapy Modality | Evidence/ Trial Number |
| Fecal Microbiome Transfer (FMT) | Philips 2017; Bajaj 2021; NCT04758806 |
| Bovine Colostrum | NCT01968382; NCT02473341 |
| F-652 (recombinant IL-22) | NCT02655510 |
| Canakinumab (IL-1 Inhibitor) | NCT03775109 |
| Selonsertib (ASK-1 Inhibitor) | NCT02854631 |
(9) AUD and abstinence
Abstinence remains the factor with the highest impact on morbidity and mortality in patients with AUD and alcoholic liver disease. There is consensus that these patients should be managed within a multidisciplinary team that includes addiction specialists (Arab 2022). Identification of underlying psychiatric comorbidities is essential. For AUD, a variety of drugs with alcohol anticraving properties are available but should be used with caution in the setting of alcoholic hepatitis and its sequelae; baclofen is considered safe in ALD, while disulfiram, acamprosate, and naltrexone should all be avoided in patients with advanced liver disease (Arab 2022).
Rehabilitation should be initiated as soon as possible. Initiation during in-patient treatment of alcoholic hepatitis or within 30 days of initial presentation significantly reduces readmission, alcohol relapse and death (Peeraphatdit 2020) and while most outpatient hepatologists remain reluctant to prescribe medical treatment for AUD, their out-patient use has been associated with lower rates of disease progression (Vannier 2022, Vannier 2022).
Summary
Alcoholic hepatitis is a clinical syndrome for which diagnosis is established based on patient history of heavy alcohol consumption, jaundice, signs of liver failure, and the absence of other reasonable causes of liver injury. A liver biopsy may be helpful but is not required either to determine the diagnosis or enable prognosis. Abstinence is the most important factor for recovery and rehabilitation and should be initiated during hospitalisation. Patients with severe alcoholic hepatitis (Maddrey’s discriminant function >32 or MELD score >21) should receive corticosteroid therapy in the absence of contraindications. Benefit from corticosteroid therapy should be evaluated after four to seven days using the Lille model (Louvet 2007). Traditionally, pentoxifylline has been employed as a secondary treatment option, but its use is not universally recommended without clear evidence for impact on mortality. In non-steroid responsive alcoholic hepatitis, carefully selected patients may benefit from liver transplantation. Emerging therapies aiming to improve intestinal dysbiosis as an important element in the pathogenesis of alcoholic hepatitis seem promising and deserve further investigation.
References
Collaborators GBDA. Alcohol use and burden for 195 countries and territories, 1990-2016: a systematic analysis for the Global Burden of Disease Study 2016. Lancet. Sep 22 2018;392(10152):1015-1035. doi:10.1016/S0140-6736(18)31310-2
Stein E, Cruz-Lemini M, Altamirano J, et al. Heavy daily alcohol intake at the population level predicts the weight of alcohol in cirrhosis burden worldwide. J Hepatol. Nov 2016;65(5):998-1005. doi:10.1016/j.jhep.2016.06.018
Singal AK, Mathurin P. Diagnosis and Treatment of Alcohol-Associated Liver Disease: A Review. JAMA. Jul 13 2021;326(2):165-176. doi:10.1001/jama.2021.7683
Grossman ER, Benjamin-Neelon SE, Sonnenschein S. Alcohol Consumption during the COVID-19 Pandemic: A Cross-Sectional Survey of US Adults. Int J Environ Res Public Health. Dec 9 2020;17(24)doi:10.3390/ijerph17249189
Julien J, Ayer T, Tapper EB, Barbosa C, Dowd WN, Chhatwal J. Effect of increased alcohol consumption during COVID-19 pandemic on alcohol-associated liver disease: A modeling study. Hepatology. Jun 2022;75(6):1480-1490. doi:10.1002/hep.32272
Drinking Levels Defined. NIAAA. 2018. https://www.niaaa.nih.gov/alcohol-health/overview-alcohol-consumption/moderate-binge-drinking
Collaborators GBDA. Population-level risks of alcohol consumption by amount, geography, age, sex, and year: a systematic analysis for the Global Burden of Disease Study 2020. Lancet. Jul 16 2022;400(10347):185-235. doi:10.1016/S0140-6736(22)00847-9
Hagstrom H, Hemmingsson T, Discacciati A, Andreasson A. Alcohol consumption in late adolescence is associated with an increased risk of severe liver disease later in life. J Hepatol. Mar 2018;68(3):505-510. doi:10.1016/j.jhep.2017.11.019
O'Connor EA, Perdue LA, Senger CA, et al. Screening and Behavioral Counseling Interventions to Reduce Unhealthy Alcohol Use in Adolescents and Adults: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA. Nov 13 2018;320(18):1910-1928. doi:10.1001/jama.2018.12086
Bohn MJ, Babor TF, Kranzler HR. The Alcohol Use Disorders Identification Test (AUDIT): validation of a screening instrument for use in medical settings. J Stud Alcohol. Jul 1995;56(4):423-32. doi:10.15288/jsa.1995.56.423
Bataller R, Arab JP, Shah VH. Alcohol-Associated Hepatitis. N Engl J Med. Dec 29 2022;387(26):2436-2448. doi:10.1056/NEJMra2207599
Sultatos LG, Pastino GM, Rosenfeld CA, Flynn EJ. Incorporation of the genetic control of alcohol dehydrogenase into a physiologically based pharmacokinetic model for ethanol in humans. Toxicol Sci. Mar 2004;78(1):20-31. doi:10.1093/toxsci/kfh057
You M, Crabb DW. Recent advances in alcoholic liver disease II. Minireview: molecular mechanisms of alcoholic fatty liver. Am J Physiol Gastrointest Liver Physiol. Jul 2004;287(1):G1-6. doi:10.1152/ajpgi.00056.2004
Fischer M, You M, Matsumoto M, Crabb DW. Peroxisome proliferator-activated receptor alpha (PPARalpha) agonist treatment reverses PPARalpha dysfunction and abnormalities in hepatic lipid metabolism in ethanol-fed mice. J Biol Chem. Jul 25 2003;278(30):27997-8004. doi:10.1074/jbc.M302140200
Ji C, Chan C, Kaplowitz N. Predominant role of sterol response element binding proteins (SREBP) lipogenic pathways in hepatic steatosis in the murine intragastric ethanol feeding model. J Hepatol. Nov 2006;45(5):717-24. doi:10.1016/j.jhep.2006.05.009
You M, Matsumoto M, Pacold CM, Cho WK, Crabb DW. The role of AMP-activated protein kinase in the action of ethanol in the liver. Gastroenterology. Dec 2004;127(6):1798-808. doi:10.1053/j.gastro.2004.09.049
Schaffert CS, Duryee MJ, Hunter CD, et al. Alcohol metabolites and lipopolysaccharide: roles in the development and/or progression of alcoholic liver disease. World J Gastroenterol. Mar 14 2009;15(10):1209-18. doi:10.3748/wjg.15.1209
Hritz I, Mandrekar P, Velayudham A, et al. The critical role of toll-like receptor (TLR) 4 in alcoholic liver disease is independent of the common TLR adapter MyD88. Hepatology. Oct 2008;48(4):1224-31. doi:10.1002/hep.22470
Meagher EA, Barry OP, Burke A, et al. Alcohol-induced generation of lipid peroxidation products in humans. J Clin Invest. Sep 1999;104(6):805-13. doi:10.1172/JCI5584
Wu D, Cederbaum AI. Oxidative stress and alcoholic liver disease. Semin Liver Dis. May 2009;29(2):141-54. doi:10.1055/s-0029-1214370
Bailey SM, Cunningham CC. Acute and chronic ethanol increases reactive oxygen species generation and decreases viability in fresh, isolated rat hepatocytes. Hepatology. Nov 1998;28(5):1318-26. doi:10.1002/hep.510280521
Kamimura S, Gaal K, Britton RS, Bacon BR, Triadafilopoulos G, Tsukamoto H. Increased 4-hydroxynonenal levels in experimental alcoholic liver disease: association of lipid peroxidation with liver fibrogenesis. Hepatology. Aug 1992;16(2):448-53. doi:10.1002/hep.1840160225
Pessayre D, Mansouri A, Haouzi D, Fromenty B. Hepatotoxicity due to mitochondrial dysfunction. Cell Biol Toxicol. 1999;15(6):367-73. doi:10.1023/a:1007649815992
Lu Y, Zhuge J, Wang X, Bai J, Cederbaum AI. Cytochrome P450 2E1 contributes to ethanol-induced fatty liver in mice. Hepatology. May 2008;47(5):1483-94. doi:10.1002/hep.22222
Ji C, Kaplowitz N. Betaine decreases hyperhomocysteinaemia, endoplasmic reticulum stress, and liver injury in alcohol-fed mice. Gastroenterology. May 2003;124(5):1488-99. doi:10.1016/s0016-5085(03)00276-2
Yin M, Gabele E, Wheeler MD, et al. Alcohol-induced free radicals in mice: direct toxicants or signaling molecules? Hepatology. Nov 2001;34(5):935-42. doi:10.1053/jhep.2001.28888
Teschke R. Alcoholic Liver Disease: Alcohol Metabolism, Cascade of Molecular Mechanisms, Cellular Targets, and Clinical Aspects. Biomedicines. Nov 12 2018;6(4)doi:10.3390/biomedicines6040106
Wu D, Cederbaum AI. Alcohol, oxidative stress, and free radical damage. Alcohol Res Health. 2003;27(4):277-84.
Szabo G. Gut-liver axis in alcoholic liver disease. Gastroenterology. Jan 2015;148(1):30-6. doi:10.1053/j.gastro.2014.10.042
Keshavarzian A, Holmes EW, Patel M, Iber F, Fields JZ, Pethkar S. Leaky gut in alcoholic cirrhosis: a possible mechanism for alcohol-induced liver damage. Am J Gastroenterol. Jan 1999;94(1):200-7. doi:10.1111/j.1572-0241.1999.00797.x
Dunagan M, Chaudhry K, Samak G, Rao RK. Acetaldehyde disrupts tight junctions in Caco-2 cell monolayers by a protein phosphatase 2A-dependent mechanism. Am J Physiol Gastrointest Liver Physiol. Dec 15 2012;303(12):G1356-64. doi:10.1152/ajpgi.00526.2011
Bajaj JS. Alcohol, liver disease and the gut microbiota. Nat Rev Gastroenterol Hepatol. Apr 2019;16(4):235-246. doi:10.1038/s41575-018-0099-1
Lang S, Duan Y, Liu J, et al. Intestinal Fungal Dysbiosis and Systemic Immune Response to Fungi in Patients With Alcoholic Hepatitis. Hepatology. Feb 2020;71(2):522-538. doi:10.1002/hep.30832
Jiang L, Lang S, Duan Y, et al. Intestinal Virome in Patients With Alcoholic Hepatitis. Hepatology. Dec 2020;72(6):2182-2196. doi:10.1002/hep.31459
Chen P, Starkel P, Turner JR, Ho SB, Schnabl B. Dysbiosis-induced intestinal inflammation activates tumour necrosis factor receptor I and mediates alcoholic liver disease in mice. Hepatology. Mar 2015;61(3):883-94. doi:10.1002/hep.27489
Duan Y, Llorente C, Lang S, et al. Bacteriophage targeting of gut bacterium attenuates alcoholic liver disease. Nature. Nov 2019;575(7783):505-511. doi:10.1038/s41586-019-1742-x
Bala S, Marcos M, Gattu A, Catalano D, Szabo G. Acute binge drinking increases serum endotoxin and bacterial DNA levels in healthy individuals. PLoS One. 2014;9(5):e96864. doi:10.1371/journal.pone.0096864
Uesugi T, Froh M, Arteel GE, Bradford BU, Thurman RG. Toll-like receptor 4 is involved in the mechanism of early alcohol-induced liver injury in mice. Hepatology. Jul 2001;34(1):101-8. doi:10.1053/jhep.2001.25350
Petrasek J, Mandrekar P, Szabo G. Toll-like receptors in the pathogenesis of alcoholic liver disease. Gastroenterol Res Pract. 2010;2010doi:10.1155/2010/710381
Yoseph BP, Breed E, Overgaard CE, et al. Chronic alcohol ingestion increases mortality and organ injury in a murine model of septic peritonitis. PLoS One. 2013;8(5):e62792. doi:10.1371/journal.pone.0062792
Adachi Y, Bradford BU, Gao W, Bojes HK, Thurman RG. Inactivation of Kupffer cells prevents early alcohol-induced liver injury. Hepatology. Aug 1994;20(2):453-60.
Bird GL, Sheron N, Goka AK, Alexander GJ, Williams RS. Increased plasma tumour necrosis factor in severe alcoholic hepatitis. Ann Intern Med. Jun 15 1990;112(12):917-20. doi:10.7326/0003-4819-112-12-917
Yin M, Wheeler MD, Kono H, et al. Essential role of tumour necrosis factor alpha in alcohol-induced liver injury in mice. Gastroenterology. Oct 1999;117(4):942-52. doi:10.1016/s0016-5085(99)70354-9
Enomoto N, Yamashina S, Kono H, et al. Development of a new, simple rat model of early alcohol-induced liver injury based on sensitisation of Kupffer cells. Hepatology. Jun 1999;29(6):1680-9. doi:10.1002/hep.510290633
Becker U, Deis A, Sorensen TI, et al. Prediction of risk of liver disease by alcohol intake, sex, and age: a prospective population study. Hepatology. May 1996;23(5):1025-9. doi:10.1002/hep.510230513
Sato N, Lindros KO, Baraona E, et al. Sex difference in alcohol-related organ injury. Alcohol Clin Exp Res. May 2001;25(5 Suppl ISBRA):40S-45S. doi:10.1097/00000374-200105051-00007
Romeo S, Kozlitina J, Xing C, et al. Genetic variation in PNPLA3 confers susceptibility to nonalcoholic fatty liver disease. Nat Genet. Dec 2008;40(12):1461-5. doi:10.1038/ng.257
Yuan X, Waterworth D, Perry JR, et al. Population-based genome-wide association studies reveal six loci influencing plasma levels of liver enzymes. Am J Hum Genet. Oct 2008;83(4):520-8. doi:10.1016/j.ajhg.2008.09.012
Baclig MO, Lozano-Kuhne JP, Mapua CA, Gopez-Cervantes J, Natividad FF, St Luke's Liver Diseases Study G. Genetic variation I148M in patatin-like phospholipase 3 gene and risk of non-alcoholic fatty liver disease among Filipinos. Int J Clin Exp Med. 2014;7(8):2129-36.
DiStefano JK, Kingsley C, Craig Wood G, et al. Genome-wide analysis of hepatic lipid content in extreme obesity. Acta Diabetol. Apr 2015;52(2):373-82. doi:10.1007/s00592-014-0654-3
Buch S, Stickel F, Trepo E, et al. A genome-wide association study confirms PNPLA3 and identifies TM6SF2 and MBOAT7 as risk loci for alcohol-related cirrhosis. Nat Genet. Dec 2015;47(12):1443-8. doi:10.1038/ng.3417
Trepo E, Nahon P, Bontempi G, et al. Association between the PNPLA3 (rs738409 C>G) variant and hepatocellular carcinoma: Evidence from a meta-analysis of individual participant data. Hepatology. Jun 2014;59(6):2170-7. doi:10.1002/hep.26767
Shen JH, Li YL, Li D, Wang NN, Jing L, Huang YH. The rs738409 (I148M) variant of the PNPLA3 gene and cirrhosis: a meta-analysis. J Lipid Res. Jan 2015;56(1):167-75. doi:10.1194/jlr.M048777
Burza MA, Molinaro A, Attilia ML, et al. PNPLA3 I148M (rs738409) genetic variant and age at onset of at-risk alcohol consumption are independent risk factors for alcoholic cirrhosis. Liver Int. Apr 2014;34(4):514-20. doi:10.1111/liv.12310
Valenti L, Dongiovanni P, Ginanni Corradini S, Burza MA, Romeo S. PNPLA3 I148M variant and hepatocellular carcinoma: a common genetic variant for a rare disease. Dig Liver Dis. Aug 2013;45(8):619-24. doi:10.1016/j.dld.2012.12.006
Trepo E, Guyot E, Ganne-Carrie N, et al. PNPLA3 (rs738409 C>G) is a common risk variant associated with hepatocellular carcinoma in alcoholic cirrhosis. Hepatology. Apr 2012;55(4):1307-8. doi:10.1002/hep.25518
Nault JC, Nahon P. Genetic predisposition to hepatocellular carcinoma in alcoholic cirrhosis: the NCAN-PNPLA3-lipid connection? J Hepatol. Nov 2014;61(5):971-2. doi:10.1016/j.jhep.2014.08.001
Falleti E, Cussigh A, Cmet S, Fabris C, Toniutto P. PNPLA3 rs738409 and TM6SF2 rs58542926 variants increase the risk of hepatocellular carcinoma in alcoholic cirrhosis. Dig Liver Dis. Jan 2016;48(1):69-75. doi:10.1016/j.dld.2015.09.009
Stickel F, Hampe J, Trepo E, Datz C, Romeo S. PNPLA3 genetic variation in alcoholic steatosis and liver disease progression. Hepatobiliary Surg Nutr. Jun 2015;4(3):152-60. doi:10.3978/j.issn.2304-3881.2014.11.04
Strnad P, Buch S, Hamesch K, et al. Heterozygous carriage of the alpha1-antitrypsin Pi*Z variant increases the risk to develop liver cirrhosis. Gut. Jun 2019;68(6):1099-1107. doi:10.1136/gutjnl-2018-316228
Sujan R, Cruz-Lemini M, Altamirano J, et al. A Validated Score Predicts Acute Kidney Injury and Survival in Patients With Alcoholic Hepatitis. Liver Transpl. Dec 2018;24(12):1655-1664. doi:10.1002/lt.25328
Crabb DW, Bataller R, Chalasani NP, et al. Standard Definitions and Common Data Elements for Clinical Trials in Patients With Alcoholic Hepatitis: Recommendation From the NIAAA Alcoholic Hepatitis Consortia. Gastroenterology. Apr 2016;150(4):785-90. doi:10.1053/j.gastro.2016.02.042
Clemente-Sanchez A, Oliveira-Mello A, Bataller R. Moderate Alcoholic Hepatitis. Clin Liver Dis. Aug 2021;25(3):537-555. doi:10.1016/j.cld.2021.03.001
Kleiner DE, Brunt EM. Nonalcoholic fatty liver disease: pathologic patterns and biopsy evaluation in clinical research. Semin Liver Dis. Feb 2012;32(1):3-13. doi:10.1055/s-0032-1306421
Altamirano J, Miquel R, Katoonizadeh A, et al. A histologic scoring system for prognosis of patients with alcoholic hepatitis. Gastroenterology. May 2014;146(5):1231-9 e1-6. doi:10.1053/j.gastro.2014.01.018
Lackner C, Stauber RE, Davies S, et al. Development and prognostic relevance of a histologic grading and staging system for alcohol-related liver disease. J Hepatol. Oct 2021;75(4):810-819. doi:10.1016/j.jhep.2021.05.029
Cohen SM, Ahn J. Review article: the diagnosis and management of alcoholic hepatitis. Aliment Pharmacol Ther. Jul 2009;30(1):3-13. doi:10.1111/j.1365-2036.2009.04002.x
Arab JP, Diaz LA, Baeza N, et al. Identification of optimal therapeutic window for steroid use in severe alcohol-associated hepatitis: A worldwide study. J Hepatol. Nov 2021;75(5):1026-1033. doi:10.1016/j.jhep.2021.06.019
Maddrey WC, Boitnott JK, Bedine MS, Weber FL, Jr., Mezey E, White RI, Jr. Corticosteroid therapy of alcoholic hepatitis. Gastroenterology. Aug 1978;75(2):193-9.
Forrest EH, Morris AJ, Stewart S, et al. The Glasgow alcoholic hepatitis score identifies patients who may benefit from corticosteroids. Gut. Dec 2007;56(12):1743-6. doi:10.1136/gut.2006.099226
Dominguez M, Rincon D, Abraldes JG, et al. A new scoring system for prognostic stratification of patients with alcoholic hepatitis. Am J Gastroenterol. Nov 2008;103(11):2747-56. doi:10.1111/j.1572-0241.2008.02104.x
Dunn W, Jamil LH, Brown LS, et al. MELD accurately predicts mortality in patients with alcoholic hepatitis. Hepatology. Feb 2005;41(2):353-8. doi:10.1002/hep.20503
Srikureja W, Kyulo NL, Runyon BA, Hu KQ. MELD score is a better prognostic model than Child-Turcotte-Pugh score or Discriminant Function score in patients with alcoholic hepatitis. J Hepatol. May 2005;42(5):700-6. doi:10.1016/j.jhep.2004.12.022
Morales-Arraez D, Ventura-Cots M, Altamirano J, et al. The MELD Score Is Superior to the Maddrey Discriminant Function Score to Predict Short-Term Mortality in Alcohol-Associated Hepatitis: A Global Study. Am J Gastroenterol. Feb 1 2022;117(2):301-310. doi:10.14309/ajg.0000000000001596
Forrest EH, Atkinson SR, Richardson P, et al. Application of prognostic scores in the STOPAH trial: Discriminant function is no longer the optimal scoring system in alcoholic hepatitis. J Hepatol. Mar 2018;68(3):511-518. doi:10.1016/j.jhep.2017.11.017
Louvet A, Naveau S, Abdelnour M, et al. The Lille model: a new tool for therapeutic strategy in patients with severe alcoholic hepatitis treated with steroids. Hepatology. Jun 2007;45(6):1348-54. doi:10.1002/hep.21607
Garcia-Saenz-de-Sicilia M, Duvoor C, Altamirano J, et al. A Day-4 Lille Model Predicts Response to Corticosteroids and Mortality in Severe Alcoholic Hepatitis. Am J Gastroenterol. Feb 2017;112(2):306-315. doi:10.1038/ajg.2016.539
Imperiale TF, McCullough AJ. Do corticosteroids reduce mortality from alcoholic hepatitis? A meta-analysis of the randomised trials. Ann Intern Med. Aug 15 1990;113(4):299-307. doi:10.7326/0003-4819-113-4-299
Imperiale TF, O'Connor JB, McCullough AJ. Corticosteroids are effective in patients with severe alcoholic hepatitis. Am J Gastroenterol. Oct 1999;94(10):3066-8. doi:10.1111/j.1572-0241.1999.03066.x
Rambaldi A, Saconato HH, Christensen E, Thorlund K, Wetterslev J, Gluud C. Systematic review: glucocorticosteroids for alcoholic hepatitis--a Cochrane Hepato-Biliary Group systematic review with meta-analyses and trial sequential analyses of randomised clinical trials. Aliment Pharmacol Ther. Jun 2008;27(12):1167-78. doi:10.1111/j.1365-2036.2008.03685.x
Christensen E, Gluud C. Glucocorticosteroids are not effective in alcoholic hepatitis. Am J Gastroenterol. Oct 1999;94(10):3065-6. doi:10.1111/j.1572-0241.1999.03065.x
Mathurin P, Mendenhall CL, Carithers RL, Jr., et al. Corticosteroids improve short-term survival in patients with severe alcoholic hepatitis (AH): individual data analysis of the last three randomised placebo controlled double blind trials of corticosteroids in severe AH. J Hepatol. Apr 2002;36(4):480-7. doi:10.1016/s0168-8278(01)00289-6
Mathurin P, Abdelnour M, Ramond MJ, et al. Early change in bilirubin levels is an important prognostic factor in severe alcoholic hepatitis treated with prednisolone. Hepatology. Dec 2003;38(6):1363-9. doi:10.1016/j.hep.2003.09.038
Thursz MR, Forrest EH, Ryder S, investigators S. Prednisolone or Pentoxifylline for Alcoholic Hepatitis. N Engl J Med. Jul 16 2015;373(3):282-3. doi:10.1056/NEJMc1506342
Barnes PJ, Karin M. Nuclear factor-kappaB: a pivotal transcription factor in chronic inflammatory diseases. N Engl J Med. Apr 10 1997;336(15):1066-71. doi:10.1056/NEJM199704103361506
Taieb J, Mathurin P, Elbim C, et al. Blood neutrophil functions and cytokine release in severe alcoholic hepatitis: effect of corticosteroids. J Hepatol. Apr 2000;32(4):579-86. doi:10.1016/s0168-8278(00)80219-6
Spahr L, Rubbia-Brandt L, Pugin J, et al. Rapid changes in alcoholic hepatitis histology under steroids: correlation with soluble intercellular adhesion molecule-1 in hepatic venous blood. J Hepatol. Nov 2001;35(5):582-9. doi:10.1016/s0168-8278(01)00190-8
O'Shea R, McCullough AJ. Steroids or cocktails for alcoholic hepatitis. J Hepatol. Apr 2006;44(4):633-6. doi:10.1016/j.jhep.2006.01.011
Lucey MR, Mathurin P, Morgan TR. Alcoholic hepatitis. N Engl J Med. Jun 25 2009;360(26):2758-69. doi:10.1056/NEJMra0805786
Akriviadis E, Botla R, Briggs W, Han S, Reynolds T, Shakil O. Pentoxifylline improves short-term survival in severe acute alcoholic hepatitis: a double-blind, placebo-controlled trial. Gastroenterology. Dec 2000;119(6):1637-48. doi:10.1053/gast.2000.20189
Park SH, Kim DJ, Kim YS, et al. Pentoxifylline vs. corticosteroid to treat severe alcoholic hepatitis: a randomised, non-inferiority, open trial. J Hepatol. Oct 2014;61(4):792-8. doi:10.1016/j.jhep.2014.05.014
Louvet A, Diaz E, Dharancy S, et al. Early switch to pentoxifylline in patients with severe alcoholic hepatitis is inefficient in non-responders to corticosteroids. J Hepatol. Mar 2008;48(3):465-70. doi:10.1016/j.jhep.2007.10.010
Sidhu SS, Goyal O, Singla P, et al. Corticosteroid plus pentoxifylline is not better than corticosteroid alone for improving survival in severe alcoholic hepatitis (COPE trial). Dig Dis Sci. Jun 2012;57(6):1664-71. doi:10.1007/s10620-012-2097-4
Mathurin P, Louvet A, Duhamel A, et al. Prednisolone with vs without pentoxifylline and survival of patients with severe alcoholic hepatitis: a randomised clinical trial. JAMA. Sep 11 2013;310(10):1033-41. doi:10.1001/jama.2013.276300
Tilg H, Jalan R, Kaser A, et al. Anti-tumour necrosis factor-alpha monoclonal antibody therapy in severe alcoholic hepatitis. J Hepatol. Apr 2003;38(4):419-25. doi:10.1016/s0168-8278(02)00442-7
Menon KV, Stadheim L, Kamath PS, et al. A pilot study of the safety and tolerability of etanercept in patients with alcoholic hepatitis. Am J Gastroenterol. Feb 2004;99(2):255-60. doi:10.1111/j.1572-0241.2004.04034.x
Sharma P, Kumar A, Sharma BC, Sarin SK. Infliximab monotherapy for severe alcoholic hepatitis and predictors of survival: an open label trial. J Hepatol. Mar 2009;50(3):584-91. doi:10.1016/j.jhep.2008.10.024
Iimuro Y, Gallucci RM, Luster MI, Kono H, Thurman RG. Antibodies to tumour necrosis factor alfa attenuate hepatic necrosis and inflammation caused by chronic exposure to ethanol in the rat. Hepatology. Dec 1997;26(6):1530-7. doi:10.1002/hep.510260621
Naveau S, Chollet-Martin S, Dharancy S, et al. A double-blind randomised controlled trial of infliximab associated with prednisolone in acute alcoholic hepatitis. Hepatology. May 2004;39(5):1390-7. doi:10.1002/hep.20206
Boetticher NC, Peine CJ, Kwo P, et al. A randomised, double-blinded, placebo-controlled multicenter trial of etanercept in the treatment of alcoholic hepatitis. Gastroenterology. Dec 2008;135(6):1953-60. doi:10.1053/j.gastro.2008.08.057
Singal AK, Bataller R, Ahn J, Kamath PS, Shah VH. ACG Clinical Guideline: Alcoholic Liver Disease. Am J Gastroenterol. Feb 2018;113(2):175-194. doi:10.1038/ajg.2017.469
European Association for the Study of L. EASL clinical practical guidelines: management of alcoholic liver disease. J Hepatol. Aug 2012;57(2):399-420. doi:10.1016/j.jhep.2012.04.004
Nguyen-Khac E, Thevenot T, Piquet MA, et al. Glucocorticoids plus N-acetylcysteine in severe alcoholic hepatitis. N Engl J Med. Nov 10 2011;365(19):1781-9. doi:10.1056/NEJMoa1101214
Phillips M, Curtis H, Portmann B, Donaldson N, Bomford A, O'Grady J. Antioxidants versus corticosteroids in the treatment of severe alcoholic hepatitis--a randomised clinical trial. J Hepatol. Apr 2006;44(4):784-90. doi:10.1016/j.jhep.2005.11.039
Stewart S, Prince M, Bassendine M, et al. A randomised trial of antioxidant therapy alone or with corticosteroids in acute alcoholic hepatitis. J Hepatol. Aug 2007;47(2):277-83. doi:10.1016/j.jhep.2007.03.027
Mezey E, Potter JJ, Rennie-Tankersley L, Caballeria J, Pares A. A randomised placebo controlled trial of vitamin E for alcoholic hepatitis. J Hepatol. Jan 2004;40(1):40-6. doi:10.1016/s0168-8278(03)00476-8
Singh V, Sharma AK, Narasimhan RL, Bhalla A, Sharma N, Sharma R. Granulocyte colony-stimulating factor in severe alcoholic hepatitis: a randomised pilot study. Am J Gastroenterol. Sep 2014;109(9):1417-23. doi:10.1038/ajg.2014.154
Marot A, Singal AK, Moreno C, Deltenre P. Granulocyte colony-stimulating factor for alcoholic hepatitis: A systematic review and meta-analysis of randomised controlled trials. JHEP Rep. Oct 2020;2(5):100139. doi:10.1016/j.jhepr.2020.100139
Mendenhall CL, Anderson S, Weesner RE, Goldberg SJ, Crolic KA. Protein-calorie malnutrition associated with alcoholic hepatitis. Veterans Administration Cooperative Study Group on Alcoholic Hepatitis. Am J Med. Feb 1984;76(2):211-22. doi:10.1016/0002-9343(84)90776-9
Mendenhall CL, Tosch T, Weesner RE, et al. VA cooperative study on alcoholic hepatitis. II: Prognostic significance of protein-calorie malnutrition. Am J Clin Nutr. Feb 1986;43(2):213-8. doi:10.1093/ajcn/43.2.213
Stickel F, Hoehn B, Schuppan D, Seitz HK. Review article: Nutritional therapy in alcoholic liver disease. Aliment Pharmacol Ther. Aug 15 2003;18(4):357-73. doi:10.1046/j.1365-2036.2003.01660.x
Cabre E, Rodriguez-Iglesias P, Caballeria J, et al. Short- and long-term outcome of severe alcohol-induced hepatitis treated with steroids or enteral nutrition: a multicenter randomised trial. Hepatology. Jul 2000;32(1):36-42. doi:10.1053/jhep.2000.8627
Alvarez MA, Cabre E, Lorenzo-Zuniga V, Montoliu S, Planas R, Gassull MA. Combining steroids with enteral nutrition: a better therapeutic strategy for severe alcoholic hepatitis? Results of a pilot study. Eur J Gastroenterol Hepatol. Nov 2004;16(12):1375-80. doi:10.1097/00042737-200412000-00023
Moreno C, Deltenre P, Senterre C, et al. Intensive Enteral Nutrition Is Ineffective for Patients With Severe Alcoholic Hepatitis Treated With Corticosteroids. Gastroenterology. Apr 2016;150(4):903-10 e8. doi:10.1053/j.gastro.2015.12.038
Singal AK, Kamath PS, Gores GJ, Shah VH. Alcoholic hepatitis: current challenges and future directions. Clin Gastroenterol Hepatol. Apr 2014;12(4):555-64; quiz e31-2. doi:10.1016/j.cgh.2013.06.013
Mathurin P, Moreno C, Samuel D, et al. Early liver transplantation for severe alcoholic hepatitis. N Engl J Med. Nov 10 2011;365(19):1790-800. doi:10.1056/NEJMoa1105703
Lee BP, Mehta N, Platt L, et al. Outcomes of Early Liver Transplantation for Patients With Severe Alcoholic Hepatitis. Gastroenterology. Aug 2018;155(2):422-430 e1. doi:10.1053/j.gastro.2018.04.009
Lee BP, Vittinghoff E, Hsu C, et al. Predicting Low Risk for Sustained Alcohol Use After Early Liver Transplant for Acute Alcoholic Hepatitis: The Sustained Alcohol Use Post-Liver Transplant Score. Hepatology. Apr 2019;69(4):1477-1487. doi:10.1002/hep.30478
Louvet A, Labreuche J, Moreno C, et al. Early liver transplantation for severe alcohol-related hepatitis not responding to medical treatment: a prospective controlled study. Lancet Gastroenterol Hepatol. May 2022;7(5):416-425. doi:10.1016/S2468-1253(21)00430-1
Germani G, Angrisani D, Addolorato G, et al. Liver transplantation for severe alcoholic hepatitis: A multicenter Italian study. Am J Transplant. Apr 2022;22(4):1191-1200. doi:10.1111/ajt.16936
Halle P, Pare P, Kaptein E, Kanel G, Redeker AG, Reynolds TB. Double-blind, controlled trial of propylthiouracil in patients with severe acute alcoholic hepatitis. Gastroenterology. May 1982;82(5 Pt 1):925-31.
Trinchet JC, Balkau B, Poupon RE, et al. Treatment of severe alcoholic hepatitis by infusion of insulin and glucagon: a multicenter sequential trial. Hepatology. Jan 1992;15(1):76-81. doi:10.1002/hep.1840150115
Bird G, Lau JY, Koskinas J, Wicks C, Williams R. Insulin and glucagon infusion in acute alcoholic hepatitis: a prospective randomised controlled trial. Hepatology. Dec 1991;14(6):1097-101.
Arab JP, Sehrawat TS, Simonetto DA, et al. An Open-Label, Dose-Escalation Study to Assess the Safety and Efficacy of IL-22 Agonist F-652 in Patients With Alcohol-associated Hepatitis. Hepatology. Aug 2020;72(2):441-453. doi:10.1002/hep.31046
Philips CA, Pande A, Shasthry SM, et al. Healthy Donor Fecal Microbiota Transplantation in Steroid-Ineligible Severe Alcoholic Hepatitis: A Pilot Study. Clin Gastroenterol Hepatol. Apr 2017;15(4):600-602. doi:10.1016/j.cgh.2016.10.029
Bajaj JS, Gavis EA, Fagan A, et al. A Randomised Clinical Trial of Fecal Microbiota Transplant for Alcohol Use Disorder. Hepatology. May 2021;73(5):1688-1700. doi:10.1002/hep.31496
Szabo G, Mitchell M, McClain CJ, et al. IL-1 receptor antagonist plus pentoxifylline and zinc for severe alcohol-associated hepatitis. Hepatology. Oct 2022;76(4):1058-1068. doi:10.1002/hep.32478
Vergis N, Patel V, Bogdanowicz K, et al. IL-1 Signal Inhibition In Alcoholic Hepatitis (ISAIAH): a study protocol for a multicentre, randomised, placebo-controlled trial to explore the potential benefits of canakinumab in the treatment of alcoholic hepatitis. Trials. Nov 11 2021;22(1):792. doi:10.1186/s13063-021-05719-2
Arab JP, Izzy M, Leggio L, Bataller R, Shah VH. Management of alcohol use disorder in patients with cirrhosis in the setting of liver transplantation. Nat Rev Gastroenterol Hepatol. Jan 2022;19(1):45-59. doi:10.1038/s41575-021-00527-0
Peeraphatdit TB, Kamath PS, Karpyak VM, et al. Alcohol Rehabilitation Within 30 Days of Hospital Discharge Is Associated With Reduced Readmission, Relapse, and Death in Patients With Alcoholic Hepatitis. Clin Gastroenterol Hepatol. Feb 2020;18(2):477-485 e5. doi:10.1016/j.cgh.2019.04.048
Vannier AGL, Przybyszewski EM, Shay J, et al. Psychotherapy for Alcohol Use Disorder Is Associated With Reduced Risk of Incident Alcohol-Associated Liver Disease. Clin Gastroenterol Hepatol. Aug 11 2022;doi:10.1016/j.cgh.2022.08.001
Vannier AGL, Shay JES, Fomin V, et al. Incidence and Progression of Alcohol-Associated Liver Disease After Medical Therapy for Alcohol Use Disorder. JAMA Netw Open. May 2 2022;5(5):e2213014. doi:10.1001/jamanetworkopen.2022.13014
Gao X, Lv F, He X, et al. Impact of the COVID-19 pandemic on liver disease-related mortality rates in the United States. J Hepatol 2023; 78(1):16-27.
